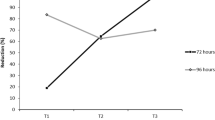
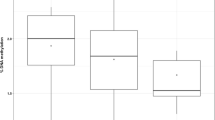
Abstract
The responses of non-target organisms to pesticide exposure are still poorly explored in what concerns the development of adjustments favouring population success. Owing to the vital role of DNA integrity, it is important to identify genome-maintenance skills and their determinant factors. Thus, the major aims of the present study were: (i) to assess the genotoxicity of the penoxsulam-based herbicide (Viper®) to the crayfish Procambarus clarkii; (ii) to understand the influence of gender and contamination history in the genotoxic responses following exposure to this herbicide; (iii) to investigate the damage mechanisms involved in putative adjustments shown by P. clarkii. Two populations were tested, one from a reference site and the other from a historically contaminated site. Specimens from both populations were exposed to Viper®, considering environmentally relevant penoxsulam concentrations (20 and 40 µg L−1) and to a model genotoxicant (EMS). Comet assay was adopted to assess the genetic damage in gills. The results disclosed the genotoxicity of the herbicide to crayfish (a non-target organism). Additionally, organisms exposed to the highest concentration of penoxsulam signalized the influence of factor “population” towards the genotoxic pressure (measured as effective DNA breaks): P2 males from the historically impacted population displayed a significantly higher susceptibly (by up to 53.98%) when compared to control, while the homologous group from the reference population presented levels similar to its respective control. When DNA lesion-repair enzymes were considered, DNA oxidation patterns suggested an increased ability of this gender (39.75% lower than negative control) to deal with this particular type of damage, namely considering pyrimidines oxidation. It is worth remarking that the influence of the exposure history on the protection/vulnerability to the penoxsulam-based herbicide was only evident in males, despite depending on the type of DNA damage: when the non-specific damage was considered, organisms from the impacted population seemed to be more vulnerable while regarding to the oxidative damage, males from the impacted population appeared to be more protected than organisms that have never been exposed to penoxsulam. Overall, the influence of factors “gender” and “contamination history” was demonstrated as well as its dependence on DNA damage type was evident. EMS groups did not present the differences between populations, reinforcing the agent-specific adjustment hypothesis.
These findings highlighted the importance of considering differential physiological backgrounds in ecogenotoxicological analysis, hence favouring the elaboration of more plausible and holistic approaches integrating the environmental risk assessment of pesticides.



Similar content being viewed by others
References
Aktar MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12. https://doi.org/10.2478/v10102-009-0001-7
Almeida C, Pereira CG, Gomes T, Cardoso C, Bebianno MJ, Cravo A (2013) Genotoxicity in two bivalve species from a coastal lagoon in the south of Portugal. Mar Environ Res 89:29–38. https://doi.org/10.1016/j.marenvres.2013.04.008
Ansoar-Rodríguez Y, Christofoletti CA, Marcato AC, Correia JE, Bueno OC, Malaspina O, Fontanetti CS (2015) Genotoxic potential of the insecticide imidacloprid in a non-target organism (Oreochromis niloticus;-Pisces). J Environ Prot 6:1360–1367. https://doi.org/10.4236/jep.2015.612118
Azqueta A, Shaposhnikov S, Collins A (2009) DNA oxidation: investigating its key role in environmental mutagenesis with the comet assay. Mutat Res 674:101–108
Benli A, Sarikaya R, Sepici-Dincel A, Selvi M, Sahin D, Erkoç F (2007) Investigation of acute toxicity of (2,4-dichlorophenoxy) acetic acid (2,4-D) herbicide on crayfish (Astacus leptodactylus Esch. 1823). Pestic Biochem Physiol 88:296–299. https://doi.org/10.1016/J.PESTBP.2007.01.004
Biggs J, Williams P, Whitfield M, Nicolet P, Brown C, Hollis J, Arnold D, Pepper T (2007) The freshwater biota of british agricultural landscapes and their sensitivity to pesticides. Agric Ecosyst Environ 122:137–148. https://doi.org/10.1016/j.agee.2006.11.013
Bleich O (2006) Exposure of population to genotoxic factors from the environment. Praha; 2017. Diplomová práce. Univerzita Karlova; 3. lékařská fakulta; Ústav hygieny. Vedoucí práce Černá; Milena.
Bolognesi C (2003) Genotoxicity of pesticides: a review of human biomonitoring studies. Mutat Res Mutat Res 543:251–272. https://doi.org/10.1016/S1383-5742(03)00015-2
Brausch JM, Smith PN (2009) Pesticide resistance from historical agricultural chemical exposure in Thamnocephalus platyurus (Crustacea: Anostraca). Environ Pollut 157:481–487. https://doi.org/10.1016/j.envpol.2008.09.010
Capela R, Raimundo J, Santos MM, Caetano M, Micaelo C, Vale C, Guimarães L, Reis-Henriques MA (2016) The use of biomarkers as integrative tools for transitional water bodies monitoring in the water framework directive context—a holistic approach in Minho river transitional waters. Sci Total Environ 539:85–96. https://doi.org/10.1016/j.scitotenv.2015.08.113
Cavas T (2011) In vivo genotoxicity evaluation of atrazine and atrazine-based herbicide on fish Carassius auratus using the micronucleus test and the comet assay. Food Chem Toxicol 49:1431–1435. https://doi.org/10.1016/j.fct.2011.03.038
Collins AR (2004) The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol 26:249–261
Dagnac T, Garcıá-Chao M, Fernadez-Lvarez M, Castro-Insua J, Garcıá-Pomar I, Llompart M (2012) Study of the presence of priority pesticides in surface water of river basins located in two areas of intensive dairy farming in the NW Spain (Galicia). Intern J Environ Anal Chem 92:995–1011. https://doi.org/10.1080/03067319.2011.603188
de la Sienra E, Armienta M, Gonsebatt M (2003) Potassium dichromate increases the micronucleus frequency in the crayfish Procambarus clarkii. Environ Pollut 126:367–370. https://doi.org/10.1016/S0269-7491(03)00249-5
Depledge MH (1994) Genotypic toxicity: implications for individuals and populations. Environ Health Perspect 102:101–104.
Desouky MMA, Abdel-Gawad H, Hegazi B (2013) Distribution, fate and histopathological effects of ethion insecticide on selected organs of the crayfish Procambarus clarkii. Food Chem Toxicol 52:42–52. https://doi.org/10.1016/j.fct.2012.10.029
EFSA (2009) Conclusion regarding the peer review of the pesticide risk assessment of the active substance penoxsulam. EFSA J 7:343r. https://doi.org/10.2903/j.efsa.2009.343r
Filho DW, Torres MA, Tribess TB, Pedrosa RC, Soares CHL (2001) Influence of season and pollution on the antioxidant defenses of the cichlid fish acará (Geophagus brasiliensis). Braz J Med Biol Res 34:719–726. https://doi.org/10.1590/S0100-879X2001000600004
Fleeger JW, Carman KR, Nisbet RM (2003) Indirect effects of contaminants in aquatic ecosystems. Sci Total Environ 317:207–233. https://doi.org/10.1016/S0048-9697(03)00141-4
Forbes VE (2000) Is hormesis an evolutionary expectation? Funct Ecol 14:12–24. https://doi.org/10.1046/j.1365-2435.2000.00392.x
Gherardi F (2006) Crayfish invading Europe: the case study of Procambarus clarkii. Mar Freshw Behav Physiol 39:175–191. https://doi.org/10.1080/10236240600869702
Gherardi F, Lazzara L (2006) Effects of the density of an invasive crayfish (Procambarus clarkii) on pelagic and surface microalgae in a Mediterranean wetland. Arch für Hydrobiol 165:401–414. https://doi.org/10.1127/0003-9136/2006/0165-0401
Gonzalez F, Nebert D (1996) Evolution of the P450 gene superfamily: animal-plant “warfare”, molecular drive and human genetic differences in drug oxidation. Trends Genet 6:182–186. https://doi.org/10.1016/0168-9525(90)90174-5
Guilherme S, Gaivão I, Santos MA, Pacheco M (2010) European eel (Anguilla anguilla) genotoxic and pro-oxidant responses following short-term exposure to Roundup®: a glyphosate-based herbicide. Mutagenesis 25:523–530
Guilherme S, Santos MA, Gaivão I, Pacheco M (2014) Are DNA-damaging effects induced by herbicide formulations (Roundup® and Garlon®) in fish transient and reversible upon cessation of exposure? Aquat Toxicol 155:213–221. https://doi.org/10.1016/j.aquatox.2014.06.007
Guilherme S, Santos MA, Gaivão I, Pacheco M (2015) Genotoxicity evaluation of the herbicide Garlon® and its active ingredient (triclopyr) in fish (Anguilla anguilla L.) using the comet assay. Environ Toxicol 30:1073–1081. https://doi.org/10.1002/tox.21980
Guilherme S, Santos M, Barroso C, Gaivão I, Pacheco M (2012) Differential genotoxicity of Roundup® formulation and its constituents in blood cells of fish (Anguilla anguilla): considerations on chemical interactions and DNA damaging mechanisms. Ecotoxicology 21:1381–1390. https://doi.org/10.1007/s10646-012-0892-5
Halliwell B.E, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford.
Hartmann A, Agurell E, Beevers C, Smith A, Speit G, Thybaud V, Tice RR (2003) Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis 18:45–51. http://dx.doi.org/10.1093/mutage/18.1.45.
Helfrich, Weigmann, Hipkins, Stinson (2009) Pesticides and aquatic animals: a guide to reducing impacts on aquatic systems. Commun. Mark Coll. Agric. Life Sci., Virginia Polytech. Inst. State Univ.
Jabusch TW, Tjeerdema RS (2008) Chemistry and fate of triazolopyrimidine sulfonamide herbicides. Springer, New York, NY, p 31–52
Jha A (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23:207–221. https://doi.org/10.1093/mutage/gen014
Klerks PL, Moreau CJ (2001) Heritability of resistance to individual contaminants and to contaminant mixtures in the sheepshead minnow (Cyprinodon variegatus). Environ Toxicol Chem 20:1746–1751. https://doi.org/10.1002/etc.5620200818
Klobučar GIV, Malev O, Šrut M, Štambuk A, Lorenzon S, Cvetković Ž, Ferrero EA, Maguire I (2012) Genotoxicity monitoring of freshwater environments using caged crayfish (Astacus leptodactylus). Chemosphere 87:62–67. https://doi.org/10.1016/j.chemosphere.2011.11.060
Kouba A, Buřič M, Kozák P (2010) Bioaccumulation and effects of heavy metals in crayfish: a review. Water Air Soil Pollut 211:5–16. https://doi.org/10.1007/s11270-009-0273-8
Lewis K, Tzilivakis J (2017) Development of a data set of pesticide dissipation rates in/on various plant matrices for the pesticide properties database (PPDB). Data 2:28. https://doi.org/10.3390/data2030028
Marques A, Custódio M, Guilherme S, Gaivão I, Santos MA, Pacheco M (2014a) Assessment of chromosomal damage induced by a deltamethrin-based insecticide in fish (Anguilla anguilla L.)—a follow-up study upon exposure and post-exposure periods. Pestic Biochem Physiol 113:40–46. https://doi.org/10.1016/j.pestbp.2014.06.003
Marques A, Guilherme S, Gaivão I, Santos MA, Pacheco M (2014b) Progression of DNA damage induced by a glyphosate-based herbicide in fish (Anguilla anguilla) upon exposure and post-exposure periods—insights into the mechanisms of genotoxicity and DNA repair. Comp Biochem Physiol C Toxicol Pharmacol 166:126–133. https://doi.org/10.1016/j.cbpc.2014.07.009
Marques A, Rego A, Guilherme S, Gaivão I, Santos MA, Pacheco M (2016) Evidences of DNA and chromosomal damage induced by the mancozeb-based fungicide Mancozan® in fish (Anguilla anguilla L.). Pestic Biochem Physiol. https://doi.org/10.1016/j.pestbp.2016.03.004
Marques CR, Gonçalves AMM, Pereira R, Gonçalves F (2012) Ecotoxicological effects of Mikado® and Viper® on algae and daphnids. Environ Toxicol 27:685–699. https://doi.org/10.1002/tox.20687
Medina MH, Correa JA, Barata C (2007) Micro-evolution due to pollution: possible consequences for ecosystem responses to toxic stress. Chemosphere 67:2105–2114. https://doi.org/10.1016/J.CHEMOSPHERE.2006.12.024
Monserrat JM, Martínez PE, Geracitano LA, Amado LL, CMG Martins, GLL Pinho, Chaves IS, Ferreira-Cravo M, Ventura-Lima J, Bianchini A (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Physiol C Toxicol Pharmacol 146:221–234. https://doi.org/10.1016/j.cbpc.2006.08.012
Mukhopadhyay I, Chowdhuri DK, Bajpayee M, Dhawan A (2004) Evaluation of in vivo genotoxicity of cypermethrin in Drosophila melanogaster using the alkaline comet assay. Mutagenesis 19:85–90. https://doi.org/10.1093/mutage/geh007
Murussi CR, Thorstenberg ML, Leitemperger J, Costa M, Clasen B, Santi A, Menezes C, Engers VK, Loro VL (2014) Toxic effects of penoxsulam herbicide in two fish species reared in Southern Brazil. Bull Environ Contam Toxicol 92:81–84. https://doi.org/10.1007/s00128-013-1137-x
Nagarathna P, Johnson Wesley M, Sriram Reddy P (2013) Review on genotoxicity, its molecular mechanisms and prevention. Int J Pharm Sci Rev Res Int J Pharm Sci Rev Res 22:236–243
Rahman MF, Mahboob M, Danadevi K, Saleha Banu B, Grover P (2002) Assessment of genotoxic effects of chloropyriphos and acephate by the comet assay in mice leucocytes. Mutat Res 516:139–147. https://doi.org/10.1016/S1383-5718(02)00033-5
Regoli (2000) Total oxyradical scavenging capacity (TOSC) in polluted and translocated mussels: a predictive biomarker of oxidative stress. Aquat Toxicol 50:351–361
Robertson KD (2001) DNA methylation, methyltransferases, and cancer. Oncogene 20:3139–3155. https://doi.org/10.1038/sj.onc.1204341
Rodrigues B, Almeida F (2005) Guia de herbicidas. Grafmark, Londrina
Sanders B, Goering P, Jenkins K (1996) The role of general and metal-specific cellular responses in protection and repair of metal-induced damage: stress proteins and metallothioneins. Technical Report, 165–187
Scalici M, Gherardi F (2007) Structure and dynamics of an invasive population of the red swamp crayfish (Procambarus clarkii) in a Mediterranean wetland. Hydrobiologia 583:309–319. https://doi.org/10.1007/s10750-007-0615-8
Sevatdal S, Horsberg TE (2003) Determination of reduced sensitivity in sea lice (Lepeophtheirus salmonis Krøyer) against the pyrethroid deltamethrin using bioassays and probit modelling. Aquaculture 218:21–31. https://doi.org/10.1016/s0044-8486(02)00339-3
Shao B, Zhu L, Dong M, Wang J, Wang J, Xie H, Zhang Q, Du Z, Zhu S (2012) DNA damage and oxidative stress induced by endosulfan exposure in zebrafish (Danio rerio). Ecotoxicology 21:1533–1540. https://doi.org/10.1007/s10646-012-0907-2
Štambuk A, Pavlica M, Malović L, Klobucšar GIV (2008) Persistence of DNA damage in the freshwater mussel Unio pictorum upon exposure to ethyl methanesulphonate and hydrogen peroxide. Environ Mol Mutagen 49:217–225. https://doi.org/10.1002/em.20376
Suárez-Serrano A, Alcaraz C, Ibáñez C, Trobajo R, Barata C (2010a) Procambarus clarkii as a bioindicator of heavy metal pollution sources in the lower Ebro River and Delta. Ecotoxicol Environ Saf 73:280–286. https://doi.org/10.1016/j.ecoenv.2009.11.001
Suárez-Serrano A, Ibáñez C, Lacorte S, Barata C (2010b) Ecotoxicological effects of rice field waters on selected planktonic species: comparison between conventional and organic farming. Ecotoxicology 19:1523–1535. https://doi.org/10.1007/s10646-010-0537-5
USEPA (2004) United States environmental protection and toxic substances agency (7501C) pesticide fact sheet name of chemical: penoxsulam reason for issuance: Conditional Registration
Van Straalen NM (2003) Ecotoxicology becomes stress ecology. Environ Sci Technol 37:324A–330A
Vioque-Fernández A, de Almeida EA, López-Barea J (2007) Esterases as pesticide biomarkers in crayfish (Procambarus clarkii, Crustacea): tissue distribution, sensitivity to model compounds and recovery from inactivation. Comp Biochem Physiol Part C Toxicol Pharmacol 145:404–412. https://doi.org/10.1016/j.cbpc.2007.01.006
Vioque-Fernández A, de Almeida EA, López-Barea J (2009) Biochemical and proteomic effects in Procambarus clarkii after chlorpyrifos or carbaryl exposure under sublethal conditions. Biomarkers 14:299–310. https://doi.org/10.1080/13547500902913211
Weber L, Carvalho L, Sá N, Silva V, Beraldini N, Souza V, Conceição M (2013) Genotoxic effects of the water-soluble fraction of heavy oil in the brackish/freshwater amphipod Quadrivisio lutzi (Gammaridea) as assessed using the comet assay. Ecotoxicology 22:642–655. https://doi.org/10.1007/s10646-013-1055-z
Weston DP, Poynton HC, Wellborn GA, Lydy MJ, Blalock BJ, Sepulveda MS, Colbourne JK (2013) Multiple origins of pyrethroid insecticide resistance across the species complex of a nontarget aquatic crustacean Hyalella azteca. Proc Natl Acad Sci 110:16532–16537. https://doi.org/10.1073/pnas.1302023110
Williams LM, Oleksiak MF (2008) Signatures of selection in natural populations adapted to chronic pollution. BMC Evol Biol 8:282. https://doi.org/10.1186/1471-2148-8-282
Zar J (1996) Biostatistical Analysis. Prentice Hall International Inc, USA
Acknowledgements
Thanks are due for the financial support to CESAM (UID/AMB/50017 - POCI-01-0145-FEDER-007638), to FCT/MCTES through national funds (PIDDAC), and the co-funding by the FEDER, within the PT2020 Partnership Agreement and Compete 2020. This work was also supported by the Post-doctoral fellowships SFRH/BPD/88947/2012 (Sofia Guilherme) and SFRH/BPD/101971/2014 (Joana Pereira).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Costa, R., Pereira, J.L., Santos, M.A. et al. The role of contamination history and gender on the genotoxic responses of the crayfish Procambarus clarkii to a penoxsulam-based herbicide. Ecotoxicology 27, 908–918 (2018). https://doi.org/10.1007/s10646-018-1948-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-018-1948-y