Abstract
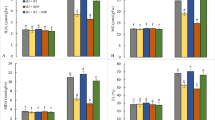
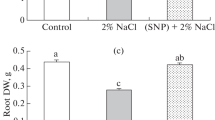
The present study investigates the possible regulatory role of exogenous nitric oxide (NO) in mitigating oxidative stress in wheat seedlings exposed to arsenic (As). Seedlings were treated with NO donor (0.25 mM sodium nitroprusside, SNP) and As (0.25 and 0.5 mM Na2HAsO4·7H2O) separately and/or in combination and grown for 72 h. Relative water content (RWC) and chlorophyll (chl) content were decreased by As treatment but proline (Pro) content was increased. The ascorbate (AsA) content was decreased significantly with increased As concentration. The imposition of As caused marked increase in the MDA and H2O2 content. The amount of reduced glutathione (GSH) and glutathione disulfide (GSSG) significantly increased with an increase in the level of As (both 0.25 and 0.5 mM), while the GSH/GSSG ratio decreased at higher concentration (0.5 mM). The ascorbate peroxidase and glutathione S-transferase activities consistently increased with an increase in the As concentration, while glutathione reductase (GR) activities increased only at 0.25 mM. The monodehydroascorbate reductase (MDHAR) and catalase (CAT) activities were not changed upon exposure to As. The activities of dehydroascorbate reductase (DHAR) and glyoxalase I (Gly I) decreased at any levels of As, while glutathione peroxidase (GPX) and glyoxalase II (Gly II) activities decreased only upon 0.5 mM As. Exogenous NO alone had little influence on the non-enzymatic and enzymatic components compared to the control seedlings. These inhibitory effects of As were markedly recovered by supplementation with SNP; that is, the treatment with SNP increased the RWC, chl and Pro contents; AsA and GSH contents and the GSH/GSSG ratio as well as the activities of MDHAR, DHAR, GR, GPX, CAT, Gly I and Gly II in the seedlings subjected to As stress. These results suggest that the exogenous application of NO rendered the plants more tolerant to As-induced oxidative damage by enhancing their antioxidant defense and glyoxalase system.





Similar content being viewed by others
Abbreviations
- AO:
-
Ascorbate oxidase
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- CAT:
-
Catalase
- CDNB:
-
1-Chloro-2,4-dinitrobenzene
- chl:
-
Chlorophyll
- DHA:
-
Dehydroascorbate
- DHAR:
-
Dehydroascorbate reductase
- DTNB:
-
5,5′-Dithio-bis(2-nitrobenzoic acid)
- EDTA:
-
Ethylenediaminetetraacetic acid
- Gly I:
-
Glyoxalase I
- Gly II:
-
Glyoxalase II
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- GPX:
-
Glutathione peroxidase
- GST:
-
Glutathione S-transferase
- MDA:
-
Malondialdehyde
- MDHA:
-
Monodehydroascorbate
- MDHAR:
-
Monodehydroascorbate reductase
- MG:
-
Methylglyoxal
- NO:
-
Nitric oxide
- NTB:
-
2-Nitro-5-thiobenzoic acid
- Pro:
-
Proline
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
- SLG:
-
S-d-Lactoylglutathione
- SNP:
-
Sodium nitroprusside
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
References
Addinsoft (2010) XLSTAT 2010: data analysis and statistics software for Microsoft Excel. Addinsoft, Paris
Anjum NA, Umar S, Iqbal M, Khan NA (2011) Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbate–glutathione cycle metabolism. Russ J Plant Physiol 58:92–99
Arnon DT (1949) Copper enzymes in isolated chloroplasts polyphenaloxidase in Beta vulgaris. Plant Physiol 24:1–15
Barrs HD, Weatherly PE (1962) A re-examination of relative turgidity for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of proline for water-stress studies. Plant Soil 39:205–207
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chao Y-Y, Hong C-Y, Kao C-H (2010) The decline in ascorbic acid content is associated with cadmium toxicity of rice seedlings. Plant Physiol Biochem 48:374–381
Chen X, Wang J, Shi Y, Zhao MQ, Chi GY (2011) Effects of cadmium on growth and photosynthetic activities in pakchoi and mustard. Bot Stud 52:41–46
Chronopoulou EG, Labrou NE (2009) Glutathione transferases: emerging multidisciplinary tools in red and green biotechnology. Recent Pat Biotechnol 3:211–223
Corpas FJ, Leterrier M, Valderrama R, Airaki M, Chaki M, Palma JM, Barroso JB (2011) Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci 181:604–611
Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55:162–167
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245:85–96
Foyer (2003) Ascorbate and glutathione metabolism in plants: H2O2-processing and signalling. In: Gitler C, Danon A (eds) Cellular implications of redox signaling. Imperial College Press, London, pp 191–212
Foyer CF, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Gao HJ, Yang HQ, Wang JX (2009) Arginine metabolism in roots and leaves of apple (Malus domestica Borkh.): the tissue-specific formation of both nitric oxide and polyamines. Sci Hort 119:147–152
Gill SS, Hasanuzzaman M, Nahar K, Macovei A, Tuteja N (2013) Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol Biochem 63:254–261
Gupta M, Sharma P, Sarin NB, Sinha AK (2009) Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosphere 74:1201–1208
Gupta KJ, Igamberdiev AU, Manjunatha G, Segu S, Moran JF, Neelawarne B, Bauwe H, Kaiser WM (2011) The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci 181:520–526
Hao GP, Zhang JH (2010) The role of nitric oxide as a bioactive signaling molecule in plants under abiotic stress. In: Hayat S, Mori M, Pichtel J, Ahmad A (eds) Nitric oxide in plant physiology. Wiley, Weinheim, pp 115–138
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment up-regulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143:1758–1776
Hasanuzzaman M, Fujita M (2012) Heavy metals in the environment: current status, toxic effects on plants and possible phytoremediation. In: Anjum NA, Pereira MA, Ahmad I, Duarte AC, Umar S, Khan NA (eds) Phytotechnologies: remediation of environmental contaminants. Taylor and Francis/CRC Press, Boca Raton, pp 7–73
Hasanuzzaman M, Hossain MA, Fujita M (2010) Physiological and biochemical mechanisms of nitric oxide induced abiotic stress tolerance in plants. Am J Plant Physiol 5:295–324
Hasanuzzaman M, Hossain MA, Fujita M (2011a) Nitric oxide modulates antioxidant defense and methylglyoxal detoxification system and reduces salinity induced damage in wheat seedling. Plant Biotecnol Rep 5:353–365
Hasanuzzaman M, Hossain MA, Fujita M (2011b) Selenium-induced upregulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Elem Res 143:1704–1721
Hasanuzzaman M, Hossain MA, da Silva JAT, Fujita M (2012a) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Venkateshwarulu B, Shanker AK, Shanker C, Mandapaka M (eds) Crop stress and its management: perspectives and strategies. Springer, Berlin, pp 261–315
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2012b) Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum) seedlings by modulating the antioxidant defense and glyoxalase system. Aust J Crop Sci 6:1314–1323
Hasanuzzaman M, Hossain MA, Fujita M (2012c) Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol Trace Elem Res 149:248–261
Hasanuzzaman M, Gill SS, Fujita M (2013) Physiological role of nitric oxide in plants grown under adverse environmental conditions. In: Tuteja N, Gill SS (eds) Plant acclimation to environmental stress. Springer, New York, pp 169–322
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Hossain MZ, Hossain MD, Fujita M (2006) Induction of pumpkin glutathione S-transferase by different stresses and its possible mechanisms. Biol Plant 50:210–218
Hsu YT, Kao CH (2004) Cd toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227–238
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in ascorbate-deficient Arabidopsis mutant. J Exp Bot 56:3041–3049
Innocenti G, Pucciariello C, Gleuher ML, Hopkins J, de Stefano M, Delledonne M, Puppo A, Baudouin E, Frendo P (2007) Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta 225:1597–1602
Islam MM, Hoque MA, Okuma E, Jannat R, Banu MNA, Jahan MS, Nakamura Y, Murata Y (2009) Proline and glycinebetaine confer cadmium tolerance on tobacco bright yellow-2 cells by increasing ascorbate-glutathione cycle enzyme activities. Biosci Biotechnol Biochem 73:2320–2323
Ismail GSM (2012) Protective role of nitric oxide against arsenic-induced damages in germinating mung bean seeds. Acta Physiol Plant 34:1303–1311
Khan I, Ahmad A, Iqbal M (2009) Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotoxicol Environ Saf 72:626–634
Kumar V, Yadav SK (2009) Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol Plant 31:261–269
Kumari A, Sheokand S, Swaraj K (2010) Nitric oxide induced alleviation of toxic effects of short term and long term Cd stress on growth, oxidative metabolism and Cd accumulation in chickpea. Braz J Plant Physiol 22:271–284
Li C, Li T, Zhang D, Jiang L, Shao Y (2013) Exogenous nitric oxide effect on fructan accumulation and FBEs expression in chilling-sensitive and chilling-resistant wheat. Environ Exp Bot 86:2–8
Liu X, Wang L, Liu L, Guo Y, Ren H (2011) Alleviating effect of exogenous nitric oxide in cucumber seedling against chilling stress. Afr J Biotechnol 10:4380–4386
Mantri N, Patade V, Penna S, Ford R, Pang E (2012) Abiotic stress responses in plants: present and future. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer, New York, pp 1–19
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Breusegem FV, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220
Mishra S, Jha AB, Dubey RS (2011) Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 248:565–577
Mustafiz A, Sahoo KK, Singla-Pareek SL, Sopory SK (2010) Metabolic engineering of glyoxalase pathway for enhancing stress tolerance in plants. Methods Mol Biol 639:95–118
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Paradiso A, Berardino R, de Pinto M, di Toppi LS, Storelli FT, de Gara L (2008) Increase in ascorbate–glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol 49:362–374
Principato GB, Rosi G, Talesa V, Govannini E, Uolila L (1987) Purification and characterization of two forms of glyoxalase II from rat liver and brain of Wistar rats. Biochem Biophys Acta 911:349–355
Rubbo H, Radi R, Anselmi D, Kirk M, Barnes S, Butler J, Eiserich JP, Freeman BA (2000) Nitric oxide reaction with lipid peroxyl radicals spares alphatocopherol during lipid peroxidation. Greater oxidant protection from the pair nitric oxide/alphatocopherol than alpha-tocopherol/ascorbate. J Biol Chem 275:10812–10818
Sandalio LM, Rodríguez-Serrano M, Gupta DK, Archilla A, Romero-Puertas MC, del Río LA (2012) Reactive oxygen species and nitric oxide in plants under cadmium stress from toxicity to signaling. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, pp 199–215
Sarma H (2011) Metal hyperaccumulation in plants: a review focusing on phytoremediation technology. J Environ Sci Technol 4:118–138
Saxena M, Deb Roy S, Singla-Pareek S-L, Sopory SK, Bhalla-Sarin N (2011) Overexpression of the glyoxalase II gene leads to enhanced salinity tolerance in Brassica juncea. The Open Plant Sci J 5:23–28
Seth CS, Remans T, Keunen E, Jozefczak M, Gielen H, Opdenakker K, Weyens N, Vangronsveld J, Cuypers A (2012) Phytoextraction of toxic metals: a central role for glutathione. Plant Cell Environ 35:334–346
Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110
Singh HP, Kaur S, Batish DR, Sharma VP, Sharma N, Kohli RK (2009) Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide 20:289–297
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2008) Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res 17:171–180
Stoeva N, Berova M, Zlatev ZL (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372
Xiong J, Fu G, Tao L, Zhu C (2010) Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch Biochem Biophys 497:13–20
Xu J, Wang W, Yin H, Liu X, Sun H, Mi Q (2010) Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326:321–330
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afr J Bot 76:167–179
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2005a) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett 579:6265–6271
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005b) Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun 337:61–67
Yadav SK, Singla-Pareek SL, Sopory SK (2008) An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metabol Drug Interact 23:51–68
Yousuf PY, Hakeem KUR, Chandna R, Ahmad P (2012) Role of glutathione reductase in plant abiotic stress. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer, New York, pp 149–158
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30:955–963
Acknowledgments
We thank Mr. Mahbub Alam and Mrs. Kamrun Nahar, Laboratory of Plant Stress Responses, Faculty of Agriculture, Kagawa University, Japan for critically reading of the manuscript. We also thank Mr. M.G. Mostofa of the same laboratory for his help in measuring the activity of glutathione reductase.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasanuzzaman, M., Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22, 584–596 (2013). https://doi.org/10.1007/s10646-013-1050-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1050-4