Summary
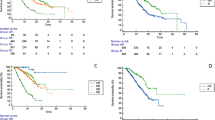
Background. We aimed to investigate the efficacy and safety of atezolizumab plus bevacizumab therapy in patients with unresectable hepatocellular carcinoma (u-HCC) based on whether they had previously received systemic therapy, as well as the association of atezolizumab plus bevacizumab with early alpha-fetoprotein (AFP) response in real-world practice. Methods. A total of 52 patients with u-HCC were treated with atezolizumab plus bevacizumab between October 2020 and April 2021. The Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST were used to evaluate radiological responses. Results. The patients received atezolizumab plus bevacizumab as 1st-line (n = 23), 2nd-line (n = 16), 3rd-line (n = 6), 4th-line (n = 3), 5th-line (n = 3), or 6th-line (n = 1) therapy. According to RECIST, the objective response rate (ORR) and disease control rate (DCR) in all patients were 15.4% and 57.7%. In the 1st-line patients, ORR and DCR based on RECIST 1.1 were 27.3% and 81.8%. The median time to progression (TTP) assessed by RECIST was significantly longer among patients receiving atezolizumab plus bevacizumab as 1st-line therapy than in patients receiving atezolizumab plus bevacizumab as later-line therapy (P < 0.001). Patients with an AFP response (reduction ≥ 20% from baseline) at 6 weeks had a significantly longer TTP assessed by RECIST than those without an AFP response (P = 0.02). Conclusion. Patients who received atezolizumab plus bevacizumab as 1st-line therapy had better clinical outcome than those who received atezolizumab plus bevacizumab in later lines. The AFP response at 6 weeks could be a predictor of disease progression.








Similar content being viewed by others
Data availability
The data presented in this study are available upon request from the corresponding author.
Code availability
Not applicable.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Bruix J, Qin S, Merle P et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56–66
Kudo M, Finn RS, Qin S et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391:1163–1173
Abou-Alfa GK, Meyer T, Cheng AL et al (2018) Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379:54–63
Park JO, Ryoo BY, Yen CJ et al (2016) Second-line ramucirumab therapy for advanced hepatocellular carcinoma (REACH): An East Asian and non-East Asian subgroup analysis. Oncotarget 7:75482–75491
Zhu AX, Kang YK, Yen CJ et al (2019) Ramucirumab in advanced hepatocellular carcinoma and elevated alpha-fetoprotein following sorafenib (REACH-2): A randomised, double-blind, placebocontrolled phase 3 trial. Lancet Oncol 20:282–296
Finn RS, Ducreux M, Qin S et al (2018) IMbrave150: A randomized phase III study of 1L atezolizumab plus bevacizumab vs sorafenib in locally advanced or metastatic hepatocellular carcinoma. J Clin Oncol 36:TPS4141–TPS4141
Kokudo N, Takemura N, Hasegawa K et al (2019) Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 49:1109–1113
Marrero JA, Kulik LM, Sirlin C et al (2018) Diagnosis, staging and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750
European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60
Johnson PJ, Berhane S, Kagebayashi C et al (2014) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 33:550–558
Hiraoka A, Kumada T, Tsuji K et al (2019) Validation of modified ALBI Grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: A multicenter analysis. Liver Cancer 8:121–129
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–445
Gordan JD, Kennedy EB, Abou-Alfa GK et al (2020) Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol 38:4317–4345
Vogel A, Martinelli E (2021) Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 32:801–805
Kuzuya T, Kawabe N, Hashimoto S et al (2021) Initial experience of atezolizumab plus bevacizumab for advanced hepatocellular carcinoma in clinical practice. Cancer Diagnosis & Prognosis 1:19–22
Hiraoka A, Kumada T, Tada T et al (2021) Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma: Early clinical experience. Cancer Rep (Hoboken) e1464
Iwamoto H, Shimose S, Noda Y et al (2021) Initial experience of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in real-world clinical practice. Cancers 13:2786
Finn RS, Qin S, Ikeda M et al (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894–1905
Terashima T, Yamashita T, Sunagozawa H et al (2018) Analysis of the liver functional reserve of patients with advanced hepatocellular carcinoma undergoing sorafenib treatment: prospects for regorafenib therapy. Hepatol Res 48:956–966
Hiraoka A, Kumada T, Atsukawa M et al (2019) Early relative change in hepatic function with Lenvatinib for Unresectable hepatocellular carcinoma. Oncology 97:334–340
Sangro B, Park J, Finn R et al (2020) CheckMate 459: long-term (minimum follow-up 33.6 months) servival outcomes with nivolmab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. Ann Oncol 31:241–242
Kudo M, Galle PR, Brandi G et al (2021) Effect of ramucirumab on ALBI grade in patients with advanced HCC: results from REACH and REACH-2. JHEP Rep 3:100215
Kuzuya T, Asahina Y, Tsuchiya K et al (2011) Early decrease in alpha-fetoprotein, but not des-gamma-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology 81:251–258
Kuzuya T, Ishigami M, Ishizu Y et al (2016) Fever within 2 weeks of Sorafenib therapy predicts favorable treatment efficacy in patients with advanced hepatocellular carcinoma. Oncology 91:261–266
Kodama K, Kawaoka T, Namba M et al (2019) Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology 97:75–81
Lee PC, Chao Y, Chen MH, Lan KH et al (2020) Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers 12:182
Aoki T, Kudo M, Ueshima K et al (2020) Exploratory analysis of lenvatinib therapy in patients with unresectable hepatocellular carcinoma who have failed prior PD−1/PD-L1 checkpoint blockade. Cancers 12:3048
Acknowledgements
We thank the patients and medical staff who contributed to this study.
Funding
This study was supported by a grant-in-aid from the Japanese Ministry of Health, Labour and Welfare and from the Japan Agency for Medical Research and Development (JP20fk0210067h0001).
Author information
Authors and Affiliations
Contributions
Study design: Yuka Hayakawa and Kaoru Tsuchiya Data collection: Yuka Hayakawa, Kaoru Tsuchiya, Masayuki Kurosaki, Yutaka Yasui, Shun Kaneko, Yuki Tanaka, Shun Ishido, Kento Inada, Sakura Kirino, Koji Yamashita, Tsubasa Nobusawa, Hiroaki Matsumoto, Tatsuya Kakegawa, Mayu Higuchi, Kenta Takaura, Shohei Tanaka, Chiaki Maeyashiki, Nobuharu Tamaki, Hiroyuki Nakanishi, Jun Itakura, Yuka Takahashi and Namiki Izumi Data analysis: Yuka Hayakawa and Kaoru Tsuchiya Data interpretation: Yuka Hayakawa, Kaoru Tsuchiya, Masayuki Kurosaki, Shun Kaneko, Yutaka Yasui, Yasuhiro Asahina, Ryuichi Okamoto and Namiki Izumi Manuscript writing: Yuka Hayakawa and Kaoru Tsuchiya Manuscript revision: Masayuki Kurosaki, Shun Kaneko, Yutaka Yasui, Yasuhiro Asahina, Ryuichi Okamoto and Namiki Izumi All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Musashino Red Cross Hospital (No. 28077).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Conflict of interest
Kaoru Tsuchiya, Masayuki Kurosaki, and Namiki Izumi received advisory board fees and honoraria from the speakers’ bureau from Bayer, Eisai, Eli Lilly Japan, and Roche. The Japanese Ministry of Health, Labour and Welfare had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hayakawa, Y., Tsuchiya, K., Kurosaki, M. et al. Early experience of atezolizumab plus bevacizumab therapy in Japanese patients with unresectable hepatocellular carcinoma in real-world practice. Invest New Drugs 40, 392–402 (2022). https://doi.org/10.1007/s10637-021-01185-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01185-4