Summary
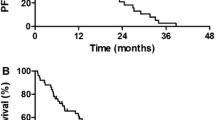
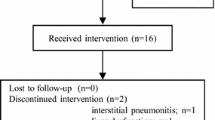
The efficacy and safety of combination therapy with erlotinib and bevacizumab in elderly patients with non-small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) gene mutations are unknown. Elderly patients aged ≥75 years old with advanced or recurrent NSCLC and EGFR mutations (exon 19 deletion or L858R mutation in exon 21) received erlotinib (150 mg, daily) and bevacizumab (15 mg/kg on day 1 of a 21-day cycle) until disease progression or the occurrence of unacceptable toxicities. The primary endpoint was progression-free survival from enrollment. Twenty-five patients were enrolled in this study, and the median age was 80 years. Fifteen (60.0%) and 10 patients (40.0%) had exon 21 L858R mutations and exon 19 deletions, respectively. The median progression-free survival from enrollment was 12.6 months [95% confidence interval (CI): 8.0–33.7 months]. The objective response rate was 88.0% [95% CI: 74.0%–99.0%], and the disease control rate was 100% [95% CI: 88.7%–100%]. Grade 3 or higher adverse events occurred in 12 patients (48.0%), and rash and nausea were the most common. Grade 3 or higher bevacizumab-related toxicities occurred in 4 (16.0%) patients, including proteinuria (n = 2), gastrointestinal perforation (n = 1) and pneumothorax (n = 1). A dose reduction of erlotinib and cessation of bevacizumab was required in 16 (64.0%) and 18 patients (72.0%), respectively. Erlotinib and bevacizumab combination therapy showed a minimal survival benefit with frequent dose reductions and/or treatment discontinuations in elderly patients with EGFR-positive NSCLC.




Similar content being viewed by others
References
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350(21):2129–2139. https://doi.org/10.1056/NEJMoa040938
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M, West Japan Oncology Group (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11(2):121–128. https://doi.org/10.1016/S1470-2045(09)70364-X
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12(8):735–742. https://doi.org/10.1016/S1470-2045(11)70184-X
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, de Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L, Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246. https://doi.org/10.1016/S1470-2045(11)70393-X
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Non-small cell lung cancer. version 3. 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (Accessed May 7 2020)
Ershler WB, Longo DL (1997) Aging and cancer: issues of basic and clinical science. J Natl Cancer Inst 89(20):1489–1497. https://doi.org/10.1093/jnci/89.20.1489
Gridelli C, Shepherd FA (2005) Chemotherapy for elderly patients with non-small cell lung Cancer: a review of the evidence. Chest. 128(2):947–957. https://doi.org/10.1378/CHEST.128.2.947
Inoue Y, Inui N, Asada K, Karayama M, Matsuda H, Yokomura K, Koshimizu N, Imokawa S, Yamada T, Shirai T, Kasamatsu N, Suda T (2015) Phase II study of erlotinib in elderly patients with non-small cell lung cancer harboring epidermal growth factor receptor mutations. Cancer Chemother Pharmacol 76(1):155–161. https://doi.org/10.1007/s00280-015-2784-x
Willett CG, Boucher Y, Di Tomaso E et al (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10(2):145–147. https://doi.org/10.1038/nm988
Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3(5):391–400. https://doi.org/10.1038/nrd1381
Ma J, Waxman DJ (2008) Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther 7(12):3670–3684. https://doi.org/10.1158/1535-7163.MCT-08-0715
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550. https://doi.org/10.1056/NEJMoa061884
Reck M, Von Pawel J, Zatloukal P et al (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiL. J Clin Oncol 27(8):1227–1234. https://doi.org/10.1200/JCO.2007.14.5466
Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, Vlahovic G, Soh CH, O'Connor P, Hainsworth J (2011) Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 377:1846–1854. https://doi.org/10.1016/S0140-6736(11)60545-X
Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, Nagase S, Okamoto I, Yamanaka T, Tajima K, Harada R, Fukuoka M, Yamamoto N (2014) Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 15:1236–1244. https://doi.org/10.1016/S1470-2045(14)70381-X
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, Nakachi I, Gemma A, Azuma K, Kurimoto F, Tsubata Y, Fujita Y, Nagashima H, Asai G, Watanabe S, Miyazaki M, Hagiwara K, Nukiwa T, Morita S, Kobayashi K, Maemondo M (2019) Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 20(5):625–635. https://doi.org/10.1016/S1470-2045(19)30035-X
Wildiers H, Guetens G, De Boeck G et al (2003) Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer 88(12):1979–1986. https://doi.org/10.1038/sj.bjc.6601005
Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CYC, Rajasekeran S, Hagedorn NL, McCarville MB, Stewart CF, Davidoff AM (2007) Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res 13(13):3942–3950. https://doi.org/10.1158/1078-0432.CCR-07-0278
Takeuchi S, Wang W, Li Q, Yamada T, Kita K, Donev IS, Nakamura T, Matsumoto K, Shimizu E, Nishioka Y, Sone S, Nakagawa T, Uenaka T, Yano S (2012) Dual inhibition of met kinase and angiogenesis to overcome HGF-induced EGFR-TKI resistance in EGFR mutant lung cancer. Am J Pathol 181(3):1034–1043. https://doi.org/10.1016/j.ajpath.2012.05.023
Laskin J, Crinò L, Felip E, Franke F, Gorbunova V, Groen H, Jiang GL, Reck M, Schneider CP (2012) Safety and efficacy of first-line bevacizumab plus chemotherapy in elderly patients with advanced or recurrent nonsquamous non-small cell lung cancer: safety of avastin in lung trial (MO19390). J Thorac Oncol 7(1):203–211. https://doi.org/10.1097/JTO.0b013e3182370e02
Ramalingam SS, Dahlberg SE, Langer CJ, Gray R, Belani CP, Brahmer JR, Sandler AB, Schiller JH, Johnson DH, Eastern Cooperative Oncology Group (2008) Outcomes for elderly, advanced-stage non-small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of eastern cooperative oncology group trial 4599. J Clin Oncol 26(1):60–64. https://doi.org/10.1200/JCO.2007.13.1144
Wozniak AJ, Kosty MP, Jahanzeb M, Brahmer JR, Spigel DR, Leon L, Fish S, Flick ED, Hazard SJ, Lynch TJ Jr (2015) Clinical outcomes in elderly patients with advanced non-small cell lung cancer: results from ARIES, a bevacizumab observational cohort study. J Clin Oncol 27(4):187–196. https://doi.org/10.1016/j.clon.2014.12.002
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA, AURA3 Investigators (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376(7):629–640. https://doi.org/10.1056/NEJMoa1612674
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS, FLAURA Investigators (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378(2):113–125. https://doi.org/10.1056/NEJMoa1713137
Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, Takamura K, Hagiwara K, Kobayashi K, Nukiwa T, Inoue A, for the North-East Japan Study Group (2020) Gefitinib alone versus gefitinib plus chemotherapy for non–small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol 38(2):115–123. https://doi.org/10.1200/JCO.19.01488
Noronha V, Patil VM, Joshi A, Menon N, Chougule A, Mahajan A, Janu A, Purandare N, Kumar R, More S, Goud S, Kadam N, Daware N, Bhattacharjee A, Shah S, Yadav A, Trivedi V, Behel V, Dutt A, Banavali SD, Prabhash K (2020) Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol 38(2):124–136. https://doi.org/10.1200/JCO.19.01154
Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, Imamura F, Yoh K, Shih JY, Au KH, Moro-Sibilot D, Enatsu S, Zimmermann A, Frimodt-Moller B, Visseren-Grul C, Reck M, Chu Q, Cortot A, Pujol JL, Moro-Sibilot D, Fabre E, Lamour C, Bischoff H, Kollmeier J, Reck M, Kimmich M, Engel-Riedel W, Hammerschmidt S, Schütte W, Syrigos K, Ho JCM, Au KH, Novello S, Ardizzoni A, Pasello G, Gregorc V, del Conte A, Galetta D, Takahashi T, Nakagawa K, Nishio M, Yoh K, Seto T, Imamura F, Kumagai T, Hotta K, Goto Y, Hosomi Y, Sakai H, Takiguchi Y, Kim YH, Kurata T, Yamaguchi H, Daga H, Okamoto I, Satouchi M, Ikeda S, Kasahara K, Atagi S, Azuma K, Kumagai T, Aoe K, Kumagai T, Aoe K, Horio Y, Yamamoto N, Tanaka H, Watanabe S, Nogami N, Ozaki T, Koyama R, Hirashima T, Kaneda H, Tomii K, Fujita Y, Seike M, Nishimura N, Kato T, Ichiki M, Saka H, Hirano K, Nakahara Y, Sugawara S, Park K, Kim SW, Min YJ, Lee HW, Kang JH, An HJ, Lee KH, Kim JS, Lee GW, Lee SY, Alexandru A, Udrea AA, Juan-Vidal Ó, Nadal-Alforja E, Gil-Bazo I, Ponce-Aix S, Paz-Ares L, Rubio-Viqueira B, Alonso Garcia M, Felip Font E, Fuentes Pradera J, Coves Sarto J, Lin MC, Su WC, Hsia TC, Chang GC, Wei YF, Chiu CH, Shih JY, Su J, Cicin I, Goksel T, Harputluoglu H, Ozyilkan O, Henning I, Popat S, Hatcher O, Mileham K, Acoba J, Garon E, Jung G, Raj M, Martin W, Dakhil S (2019) Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(12):1655–1669. https://doi.org/10.1016/S1470-2045(19)30634-5
Acknowledgments
We thank Melissa Crawford, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
All authors declare no actual or potential conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aoshima, Y., Karayama, M., Inui, N. et al. Erlotinib and bevacizumab in elderly patients ≥75 years old with non-small cell lung cancer harboring epidermal growth factor receptor mutations. Invest New Drugs 39, 210–216 (2021). https://doi.org/10.1007/s10637-020-00988-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00988-1