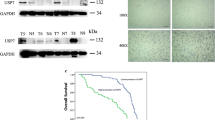
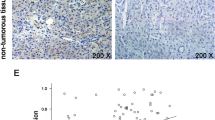
Summary
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal cancers, and most patients die within one year after diagnosis. This cancer is resistant to almost all current therapies, so there is an urgent need to identify novel druggable targets. Ubiquitin-specific protease 7 (USP7) is a deubiquitinase that functions in carcinogenesis, but its role in PDAC is unknown. Our experiments indicated that several subtypes of PDAC cells are sensitive to USP7 inhibition. In particular, pharmaceutical inhibition of USP7 by the small molecule P22077 attenuated PDAC cell growth and induced cell death in vitro and in vivo. Pharmaceutical inhibition of USP7 in P22077-resistant PDAC cells allowed them to overcome chemoresistance. Genetic silencing experiments supported the importance of USP7 in the pathogenesis of PDAC. In particular, genetic disruption of USP7 greatly reduced cell proliferation and chemoresistance in vitro and prevented PDAC growth in vivo. Protein profiling by mass spectrometry (MS) indicated USP7 was associated 4 ontology terms: translation, localization and protein transporting, nucleotide or ribonucleotide binding, and ubiquitin-dependent catabolic processes. Puromycin labeling indicated that P22077 greatly reduced protein synthesis, and transcriptional analysis indicated that P22077 significantly altered the extracellular space matrix. In summary, we provided multiple lines of evidence which indicate that USP7 plays a critical role in PDAC, and may therefore be a suitable target for treatment of this cancer.




Similar content being viewed by others
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
References
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grutzmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM (2016) Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531:47–52. https://doi.org/10.1126/science.1164368
Torres C, Grippo PJ (2018) Pancreatic cancer subtypes: a roadmap for precision medicine. Ann Med 50:277–287. https://doi.org/10.1080/07853890.2018.1453168
Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, Gray JW (2011) Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 17:500–503. https://doi.org/10.1038/nm.2344
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321:1801–1806. https://doi.org/10.1126/science.1164368
Cheng TY, Yang YC, Wang HP, Tien YW, Shun CT, Huang HY, Hsiao M, Hua KT (2018) Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene 37:1730–1742. https://doi.org/10.1038/s41388-017-0086-y
Liu PY, Xu N, Malyukova A, Scarlett CJ, Sun YT, Zhang XD, Ling D, Su SP, Nelson C, Chang DK, Koach J, Tee AE, Haber M, Norris MD, Toon C, Rooman I, Xue C, Cheung BB, Kumar S, Marshall GM, Biankin AV, Liu T (2013) The histone deacetylase SIRT2 stabilizes Myc oncoproteins. Cell Death Differ 20:503–514. https://doi.org/10.1038/cdd.2012.147
An T, Gong Y, Li X, Kong L, Ma P, Gong L, Zhu H, Yu C, Liu J, Zhou H, Mao B, Li Y (2017) USP7 inhibitor P5091 inhibits Wnt signaling and colorectal tumor growth. Biochem Pharmacol 131:29–39. https://doi.org/10.1016/j.bcp.2017.02.011
Fan YH, Cheng J, Vasudevan SA, Dou J, Zhang H, Patel RH, Ma IT, Rojas Y, Zhao Y, Yu Y, Zhang H, Shohet JM, Nuchtern JG, Kim ES, Yang J (2013) USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell Death Dis 4:e867. https://doi.org/10.1038/cddis.2013.400
Tavana O, Li D, Dai C, Lopez G, Banerjee D, Kon N, Chen C, Califano A, Yamashiro DJ, Sun H, Gu W (2016) HAUSP deubiquitinates and stabilizes N-Myc in neuroblastoma. Nat Med 22:1180–1186. https://doi.org/10.1038/nm.4180
Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu K, Fan Q, Li J, Ning T, Tian F, Li H, Sun W, Ying G, Ba Y (2019) Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene 38:2844–2859. https://doi.org/10.1038/s41388-018-0619-z
Jin Q, Martinez CA, Arcipowski KM, Zhu Y, Gutierrez-Diaz BT, Wang KK, Johnson MR, Volk AG, Wang F, Wu J, Grove C, Wang H, Sokirniy I, Thomas PM, Goo YA, Abshiru NA, Hijiya N, Peirs S, Vandamme N, Berx G, Goosens S, Marshall SA, Rendleman EJ, Takahashi YH, Wang L, Rawat R, Bartom ET, Collings CK, Van Vlierberghe P, Strikoudis A, Kelly S, Ueberheide B, Mantis C, Kandela I, Bourquin JP, Bornhauser B, Serafin V, Bresolin S, Paganin M, Accordi B, Basso G, Kelleher NL, Weinstock J, Kumar S, Crispino JD, Shilatifard A, Ntziachristos P (2019) USP7 Cooperates with NOTCH1 to Drive the Oncogenic Transcriptional Program in T-Cell Leukemia. Clin Cancer Res 25:222–239. https://doi.org/10.1158/1078-0432.CCR-18-1740
Su D, Ma S, Shan L, Wang Y, Wang Y, Cao C, Liu B, Yang C, Wang L, Tian S, Ding X, Liu X, Yu N, Song N, Liu L, Yang S, Zhang Q, Yang F, Zhang K, Shi L (2018) Ubiquitin-specific protease 7 sustains DNA damage response and promotes cervical carcinogenesis. J Clin Invest 128:4280–4296. https://doi.org/10.1172/JCI120518
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials G (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966. https://doi.org/10.1200/JCO.2006.07.9525
Hong SM, Vincent A, Kanda M, Leclerc J, Omura N, Borges M, Klein AP, Canto MI, Hruban RH, Goggins M (2012) Genome-wide somatic copy number alterations in low-grade PanINs and IPMNs from individuals with a family history of pancreatic cancer. Clin Cancer Res 18:4303–4312. https://doi.org/10.1158/10780432.CCR121075
Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, Safran H, Humblet Y, Perez Ruixo J, Ma Y, Von Hoff D (2004) Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 22:1430–1438. https://doi.org/10.1200/JCO.2004.10.112
Erez N, Truitt M, Olson P, Arron ST, Hanahan D (2010) Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 17:135–147. https://doi.org/10.1016/j.ccr.2009.12.041
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409–413. https://doi.org/10.1126/science.aan6733
Yao N, Bradley CJ, Miranda PY (2014) Mammography use after the 2009 debate. J Clin Oncol 32:4023–4024. https://doi.org/10.1200/JCO.2014.58.0191
Longley DB, Harkin DP, Johnston PG (2003) 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3:330–338. https://doi.org/10.1038/nrc1074
Manji GA, Olive KP, Saenger YM, Oberstein P (2017) Current and Emerging Therapies in Metastatic Pancreatic Cancer. Clin Cancer Res 23:1670–1678. https://doi.org/10.1158/1078-0432.CCR-16-2319
Burris HA 3, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413. https://doi.org/10.1200/JCO.1997.15.6.2403
Hertel LW, Boder GB, Kroin JS, Rinzel SM, Poore GA, Todd GC, Grindey GB (1990) Evaluation of the antitumor activity of gemcitabine (2’,2’-difluoro-2’-deoxycytidine). Cancer Res 50:4417–4422
Abal M, Andreu JM, Barasoain I (2003) Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets 3:193–203
Ishida S, Lee J, Thiele DJ, Herskowitz I (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A 99:14298–14302. https://doi.org/10.1073/pnas.162491399
Kazim S, Malafa MP, Coppola D, Husain K, Zibadi S, Kashyap T, Crochiere M, Landesman Y, Rashal T, Sullivan DM, Mahipal A (2015) Selective Nuclear Export Inhibitor KPT-330 Enhances the Antitumor Activity of Gemcitabine in Human Pancreatic Cancer. Mol Cancer Ther 14:1570–1581. https://doi.org/10.1158/15357163.MCT-15-0104
Karamitopoulou E (2019) Tumour microenvironment of pancreatic cancer: immune landscape is dictated by molecular and histopathological features. Br J Cancer. https://doi.org/10.1038/s41416-019-0479-5
Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, Babykutty S, Huang Y, Jung K, Rahbari NN, Han X, Chauhan VP, Martin JD, Kahn J, Huang P, Desphande V, Michaelson J, Michelakos TP, Ferrone CR, Soares R, Boucher Y, Fukumura D, Jain RK (2016) Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov 6:852–869. https://doi.org/10.1158/21598290.CD-15-1177
Funding
The work was supported by the National Natural Science Foundation of China (81873531; 81600386; 81572397); the Distinguished Professorship Program of Jiangsu Province to Renfang Mao; the Distinguished Professorship Program of Jiangsu Province to Yihui Fan; the Natural Science Foundation of Jiangsu Province (Grant No: BK20181458); and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX18_2406).
Author information
Authors and Affiliations
Contributions
H Chen, Y FAN, G Zhou and R Mao conceived the idea and designed the research. H Chen, X Zhu, P Ma, E Zhang and Z Wang performed in vitro experiments. H Chen and X Zhu performed mice xenograft experiments. H Chen, Rong Sun, Y Fan, G Zhou and R Mao analyzed the data. H Chen, Yihui Fan, G Zhou and R Mao wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Disclosure of potential conflicts of interest
The abstract of this manuscript was presented at the Asian Pacific Digestive Week 2019 (APDW 2019) and published in the society’s journal “Journal of Gastroenterology and Hepatology” (https://onlinelibrary.wiley.com/doi/full/10.1111/jgh.14879). All authors declare no conflict of interest.
Research involving human participants and/or animals
The protocols used for animal studies were approved by the Laboratory Animal Center of Nantong University, and all procedures followed internationally accepted principles and the Guidelines of the Animal Care and Use.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Zhu, X., Sun, R. et al. Ubiquitin-specific protease 7 is a druggable target that is essential for pancreatic cancer growth and chemoresistance. Invest New Drugs 38, 1707–1716 (2020). https://doi.org/10.1007/s10637-020-00951-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00951-0