Summary
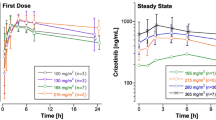
Purposes Vactosertib is a new investigational inhibitor of activin receptor-like kinase 5. The objective of this study was to characterize vactosertib pharmacokinetics that are to be applied for subsequent clinical studies. Methods Vactosertib plasma concentration-time data were obtained from a multicenter, dose-escalation, first-in-human phase 1 study conducted in patients with advanced solid tumors. Each patient orally received a fixed dose of vactosertib with the range of 30 mg to 340 mg once daily under fasted condition. Pharmacokinetic analysis was performed using a non-compartmental method. Results Pharmacokinetic data were evaluable in 29 patients. Vactosertib was rapidly absorbed after the first dose with a median time to maximum concentration (tmax) of 1.2 h (interquartile range, 0.8–1.8 h) and quickly eliminated with a median terminal half-life (t1/2) of 3.2 h (2.2–4.2 h) over the dose range studied. Such trend was also observed after repeated doses for five days (median tmax, 1.5 h; median t1/2, 3.0 h). The area under the concentration-time curve within a dosing interval increased in proportion to dose. The median values of apparent clearance and volume of distribution were 29 L/h (21–44 L/h) and 133 L (77–222 L), respectively. The median accumulation ratio after repeated once-daily doses for five days was 0.87 (0.69–1.07). Conclusions Vactosertib pharmacokinetics were dose-proportional within tested dose range with negligible accumulation when administered once daily for five days. Considering the short half-life, it seems necessary to administer vactosertib twice- or thrice-daily to maintain its concentrations above minimum effective level over a dosing interval.


Similar content being viewed by others
References
Jin CH, Krishnaiah M, Sreenu D, Subrahmanyam VB, Rao KS, Lee HJ, Park S-J, Park H-J, Lee K, Sheen YY, Kim D-K (2014) Discovery of N-((4-([1,2,4]Triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-yl)methyl)-2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-β type I receptor kinase as Cancer immunotherapeutic/Antifibrotic agent. J Med Chem 57(10):4213–4238. https://doi.org/10.1021/jm500115w
Haque S, Morris JC (2017) Transforming growth factor-β: a therapeutic target for cancer. Human Vaccines & Immunotherapeutics 13(8):1741–1750. https://doi.org/10.1080/21645515.2017.1327107
Sheen YY, Kim MJ, Park SA, Park SY, Nam JS (2013) Targeting the transforming growth factor-beta signaling in Cancer therapy. Biomol Ther 21(5):323–331. https://doi.org/10.4062/biomolther.2013.072
Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, de Gramont A (2015) Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther 147:22–31. https://doi.org/10.1016/j.pharmthera.2014.11.001
Arumugam V, Bluemn T, Wesley E, Schmidt AM, Kambayashi T, Malarkannan S, Riese MJ (2015) TCR signaling intensity controls CD8+ T cell responsiveness to TGF-beta. J Leukoc Biol 98(5):703–712. https://doi.org/10.1189/jlb.2HIMA1214-578R
Pickup M, Novitskiy S, Moses HL (2013) The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer 13(11):788–799. https://doi.org/10.1038/nrc3603
Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Canellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E (2018) TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554(7693):538–543. https://doi.org/10.1038/nature25492
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Senbabaoglu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Hoglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T (2018) TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554(7693):544–548. https://doi.org/10.1038/nature25501
Son JY, Park SY, Kim SJ, Lee SJ, Park SA, Kim MJ, Kim SW, Kim DK, Nam JS, Sheen YY (2014) EW-7197, a novel ALK-5 kinase inhibitor, potently inhibits breast to lung metastasis. Mol Cancer Ther 13(7):1704–1716. https://doi.org/10.1158/1535-7163.mct-13-0903
Yoon JH, Jung SM, Park SH, Kato M, Yamashita T, Lee IK, Sudo K, Nakae S, Han JS, Kim OH, Oh BC, Sumida T, Kuroda M, Ju JH, Jung KC, Park SH, Kim DK, Mamura M (2013) Activin receptor-like kinase5 inhibition suppresses mouse melanoma by ubiquitin degradation of Smad4, thereby derepressing eomesodermin in cytotoxic T lymphocytes. EMBO Molecular Medicine 5(11):1720–1739. https://doi.org/10.1002/emmm.201302524
Jin CH, Krishnaiah M, Sreenu D, Subrahmanyam VB, Rao KS, Lee HJ, Park SJ, Park HJ, Lee K, Sheen YY, Kim DK (2014) Discovery of N-((4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2 -yl)methyl)-2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-beta type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J Med Chem 57(10):4213–4238. https://doi.org/10.1021/jm500115w
Keedy VL, Bauer TM, Clarke JM, Hurwitz H, Baek I, Ha I, Ock C-Y, Nam SY, Kim M, Park N, Kim JY, Kim S-J (2018) Association of TGF-β responsive signature with anti-tumor effect of vactosertib, a potent, oral TGF-β receptor type I (TGFBRI) inhibitor in patients with advanced solid tumors. J Clin Oncol 36(15_suppl):3031–3031. https://doi.org/10.1200/JCO.2018.36.15_suppl.3031
Banerji U, Workman P (2016) Critical parameters in targeted drug development: the pharmacological audit trail. Semin Oncol 43(4):436–445. https://doi.org/10.1053/j.seminoncol.2016.06.001
Mathijssen R.H., Sparreboom A, Verweij J (2014) Determining the optimal dose in the development of anticancer agents, vol 11. doi:https://doi.org/10.1038/nrclinonc.2014.40
Lin JH, Lu AYH (1997) Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev 49(4):403–449
Cook N, Hansen AR, Siu LL, Abdul Razak AR (2015) Early phase clinical trials to identify optimal dosing and safety. Mol Oncol 9(5):997–1007. https://doi.org/10.1016/j.molonc.2014.07.025
Parulekar WR, Eisenhauer EA (2004) Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst 96(13):990–997
Le Tourneau C, Lee JJ, Siu LL (2009) Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 101(10):708–720. https://doi.org/10.1093/jnci/djp079
Ludden TM (1991) Nonlinear pharmacokinetics: clinical implications. Clin Pharmacokinet 20(6):429–446. https://doi.org/10.2165/00003088-199120060-00001
Zelboraf (vemurafenib) [package insert]. (2016) Genentech USA, Inc., South San Francisco CA (2016)
Verzenio (abemaciclib) [package insert]. (2017) Eli Lilly and Company, LLC Indianapolis (2017)
Tafinlar (dabrafenib) [package insert]. (2018) Novartis Pharmaceuticals Corporation, East Hanover, NJ (2018)
Gueorguieva I, Cleverly AL, Stauber A, Sada Pillay N, Rodon JA, Miles CP, Yingling JM, Lahn MM (2014) Defining a therapeutic window for the novel TGF-beta inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. Br J Clin Pharmacol 77(5):796–807
Rodon J, Carducci M, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, Brana I, Sicart E, Gueorguieva I, Cleverly A, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J (2015) Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-beta receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Investig New Drugs 33(2):357–370. https://doi.org/10.1007/s10637-014-0192-4
Flaherty KT, LoRusso PM, DeMichele A, Abramson VG, Courtney R, Randolph SS, Shaik MN, Wilner KD, O'Dwyer PJ, Schwartz GK (2012) Phase I, dose-escalation trial of the Oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced Cancer. Clin Cancer Res 18(2):568–576. https://doi.org/10.1158/1078-0432.ccr-11-0509
Ibrance (palbociclib) [package insert]. Pfizer Inc., New York, NY
Shargel L, Wu-Pong S, Yu ABC (2012) Chapter 17. Modified-release drug products. In: applied biopharmaceutics & pharmacokinetics, 6e. The McGraw-Hill Companies, New York, NY,
Kadiyala I, Tan E (2013) Formulation approaches in mitigating toxicity of orally administrated drugs. Pharm Dev Technol 18(2):305–312. https://doi.org/10.3109/10837450.2012.734516
Verstovsek S, Yeleswaram S, Hou K, Chen X, Erickson-Viitanen S (2018) Sustained-release ruxolitinib: findings from a phase 1 study in healthy subjects and a phase 2 study in patients with myelofibrosis. Hematol Oncol 36(4):701–708. https://doi.org/10.1002/hon.2544
Stuurman FE, Nuijen B, Beijnen JH, Schellens JH (2013) Oral anticancer drugs: mechanisms of low bioavailability and strategies for improvement. Clin Pharmacokinet 52(6):399–414. https://doi.org/10.1007/s40262-013-0040-2
Newman WG (2010) Pharmacogenetics: making cancer treatment safer and more effective. Springer Netherlands,
Biswal BM (2008) Current trends in the management of oral mucositis related to cancer treatment. Malaysian J Medical Sci: MJMS 15(3):4–13
Acknowledgements
We would like to acknowledge the valuable contributions of the patients who participated in the phase 1 study, their families, and study personnel. This study was supported by the Research Institutes of Pharmaceutical Science in Seoul National University. Additional support was provided by the National OncoVenture/National Cancer Center funded by Ministry of Health and Welfare, Republic of Korea (No. HI17C2196).
Funding
The phase 1 study and pharmacokinetic analysis were sponsored by MedPacto, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JIL has received research funding from MedPacto, Inc. as an advisory consultant. JMC, TMB, and VLK received grant from MedPacto, Inc. to perform of the phase 1 clinical trial as principal investigators. JMC has received research grant as a principal investigator from Bristol-Myers Squibb, Eli Lilly, Genentech, Spectrum, Adaptimmune, Medpacto, Bayer, AbbVie, and Moderna, and served as an advisory consultant for AstraZeneca, Guardant, Merck and Eli Lilly. TMB has received research grant from Daiichi Sankyo, Incyte, Mirati Therapeutics, MedImmune, Abbvie, AstraZeneca, Merck, Eli Lilly, GlaxoSmithKline, Novartis, Genentech, Deciphera, Merrimack, Immunogen, Millennium, Roche, Aileron Therapeutics, Bristol-Myers Squibb, Amgen, Onyx, Sanofi, Boehringer-Ingelheim, Astellas Pharma, Janssen, Clovis Oncology, Takeda, Karyopharm Therapeutics, Foundation Medicine, and ARMO Biosciences. VLK is a consultant for Karyopharm Therapeutics, and has research funding from Plexxicon, Eli Lilly, Daiichi Sankyo, BioMed Valley Discoveries, Immune Design, GlaxoSmithKline, TRACON Pharma, and Advenchen Laboratories. SJK and SH are a CEO and an employee of MedPacto, Inc., respectively. Other remaining authors have nothing to disclose.
Ethical approval
All procedures involving human participants performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consents were obtained from all individual participants enrolled in the phase 1 study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jung, S.Y., Hwang, S., Clarke, J.M. et al. Pharmacokinetic characteristics of vactosertib, a new activin receptor-like kinase 5 inhibitor, in patients with advanced solid tumors in a first-in-human phase 1 study. Invest New Drugs 38, 812–820 (2020). https://doi.org/10.1007/s10637-019-00835-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00835-y