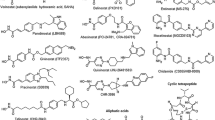
Abstract
A vast majority of lymphomas and leukaemias are results of translocations. These translocations produce various genetic and epigenetic changes that lead to oncogenesis. This opens an opportunity to use a relatively new class of anti-cancer agents, inhibitors of histone deacetylases (HDACi) to target lymphoid malignancies. Surprisingly, the rational basis for treatment of lymphomas with HDACi is far from clear, although some positive results have been obtained. Here we analyze the effect of histone deacetylase (HDAC) inhibitors on lymphoid malignancies.
Similar content being viewed by others
References
Waddington C (1942) The epigenotype. Endeavour 1:18–20
Esteller M (2011) Epigenetic changes in cancer. F1000 Biol Rep 3:9. doi:10.3410/B3-9
Nussenzweig A, Nussenzweig MC (2010) Origin of chromosomal translocations in lymphoid cancer. Cell 141(1):27–38. doi:10.1016/j.cell.2010.03.016
Korsmeyer SJ (1992) Chromosomal translocations in lymphoid malignancies reveal novel proto-oncogenes. Annu Rev Immunol 10:785–807. doi:10.1146/annurev.iy.10.040192.004033
Mitelman F, Johansson B, Mertens F (2004) Fusion genes and rearranged genes as a linear function of chromosome aberrations in cancer. Nat Genet 36(4):331–334. doi:10.1038/ng1335
Harewood L, Schutz F, Boyle S, Perry P, Delorenzi M, Bickmore WA, Reymond A (2010) The effect of translocation-induced nuclear reorganization on gene expression. Genome Res 20(5):554–564. doi:10.1101/gr.103622.109
Allinne J, Pichugin A, Iarovaia O, Klibi M, Barat A, Zlotek-Zlotkiewicz E, Markozashvili D, Petrova N, Camara-Clayette V, Ioudinkova E, Wiels J, Razin SV, Ribrag V, Lipinski M, Vassetzky YS (2014) Perinucleolar relocalization and nucleolin as crucial events in the transcriptional activation of key genes in mantle cell lymphoma. Blood 123(13):2044–2053. doi:10.1182/blood-2013-06-510511
Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara FF, Zamir I, Seiser C, Grignani F, Lazar MA, Minucci S, Pelicci PG (1998) Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 391(6669):815–818. doi:10.1038/35901
Minucci S, Maccarana M, Cioce M, De Luca P, Gelmetti V, Segalla S, Di Croce L, Giavara S, Matteucci C, Gobbi A, Bianchini A, Colombo E, Schiavoni I, Badaracco G, Hu X, Lazar MA, Landsberger N, Nervi C, Pelicci PG (2000) Oligomerization of RAR and AML1 transcription factors as a novel mechanism of oncogenic activation. Mol Cell 5(5):811–820
Lin RJ, Evans RM (2000) Acquisition of oncogenic potential by RAR chimeras in acute promyelocytic leukemia through formation of homodimers. Mol Cell 5(5):821–830
Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406(6796):641–645. doi:10.1038/35020592
Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16(1):6–21
Prioleau MN (2009) CpG islands: starting blocks for replication and transcription. PLoS Genet 5(4), e1000454. doi:10.1371/journal.pgen.1000454
Taylor KH, Briley A, Wang Z, Cheng J, Shi H, Caldwell CW (2013) Aberrant epigenetic gene regulation in lymphoid malignancies. Semin Hematol 50(1):38–47. doi:10.1053/j.seminhematol.2013.01.003
Hatzimichael E, Crook T (2013) Cancer epigenetics: new therapies and new challenges. J Drug Deliv 2013:9. doi:10.1155/2013/529312
Goelz SE, Vogelstein B, Hamilton SR, Feinberg AP (1985) Hypomethylation of DNA from benign and malignant human colon neoplasms. Science 228(4696):187–190
Kouzarides T (2007) Chromatin modifications and their function. Cell 128(4):693–705. doi:10.1016/j.cell.2007.02.005
Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y (2006) Histone demethylation by a family of JmjC domain-containing proteins. Nature 439(7078):811–816. doi:10.1038/nature04433
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119(7):941–953. doi:10.1016/j.cell.2004.12.012
Rege-Cambrin G, Giugliano E, Michaux L, Stul M, Scaravaglio P, Serra A, Saglio G, Hagemeijer A (2005) Trisomy 11 in myeloid malignancies is associated with internal tandem duplication of both MLL and FLT3 genes. Haematologica 90(2):262–264
Simon JA, Lange CA (2008) Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res 647(1–2):21–29. doi:10.1016/j.mrfmmm.2008.07.010
Sneeringer CJ, Scott MP, Kuntz KW, Knutson SK, Pollock RM, Richon VM, Copeland RA (2010) Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc Natl Acad Sci U S A 107(49):20980–20985. doi:10.1073/pnas.1012525107
Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV, Waghorn K, Zoi K, Ross FM, Reiter A, Hochhaus A, Drexler HG, Duncombe A, Cervantes F, Oscier D, Boultwood J, Grand FH, Cross NC (2010) Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet 42(8):722–726. doi:10.1038/ng.621
Nikoloski G, Langemeijer SM, Kuiper RP, Knops R, Massop M, Tonnissen ER, van der Heijden A, Scheele TN, Vandenberghe P, de Witte T, van der Reijden BA, Jansen JH (2010) Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet 42(8):665–667. doi:10.1038/ng.620
Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, Felletar I, Volkmer R, Muller S, Pawson T, Gingras AC, Arrowsmith CH, Knapp S (2012) Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 149(1):214–231. doi:10.1016/j.cell.2012.02.013
Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK (2005) Global histone modification patterns predict risk of prostate cancer recurrence. Nature 435(7046):1262–1266. doi:10.1038/nature03672
Hassler MR, Schiefer AI, Egger G (2013) Combating the epigenome: epigenetic drugs against non-Hodgkin’s lymphoma. Epigenomics 5(4):397–415. doi:10.2217/epi.13.39
Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, Trifonov V, Kasper LH, Lerach S, Tang H, Ma J, Rossi D, Chadburn A, Murty VV, Mullighan CG, Gaidano G, Rabadan R, Brindle PK, Dalla-Favera R (2011) Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 471(7337):189–195. doi:10.1038/nature09730
Dawson MA, Kouzarides T (2012) Cancer epigenetics: from mechanism to therapy. Cell 150(1):12–27. doi:10.1016/j.cell.2012.06.013
Witt O, Deubzer HE, Milde T, Oehme I (2009) HDAC family: what are the cancer relevant targets? Cancer Lett 277(1):8–21. doi:10.1016/j.canlet.2008.08.016
Kim HJ, Bae SC (2011) Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res 3(2):166–179
Kristensen LS, Nielsen HM, Hansen LL (2009) Epigenetics and cancer treatment. Eur J Pharmacol 625(1–3):131–142. doi:10.1016/j.ejphar.2009.10.011
West AC, Johnstone RW (2014) New and emerging HDAC inhibitors for cancer treatment. J Clin Invest 124(1):30–39. doi:10.1172/JCI69738
Min SK, Koh YH, Park Y, Kim HJ, Seo J, Park HR, Cho SJ, Kim IS (2012) Expression of HAT1 and HDAC1, 2, 3 in diffuse large B-cell lymphomas, peripheral T-cell lymphomas, and NK/T-cell lymphomas. Korean J Pathol 46(2):142–150. doi:10.4132/KoreanJPathol.2012.46.2.142
Heideman MR, Wilting RH, Yanover E, Velds A, de Jong J, Kerkhoven RM, Jacobs H, Wessels LF, Dannenberg JH (2013) Dosage-dependent tumor suppression by histone deacetylases 1 and 2 through regulation of c-Myc collaborating genes and p53 function. Blood 121(11):2038–2050. doi:10.1182/blood-2012-08-450916
Lee SH, Yoo C, Im S, Jung JH, Choi HJ, Yoo J (2014) Expression of histone deacetylases in diffuse large B-cell lymphoma and its clinical significance. Int J Med Sci 11(10):994–1000. doi:10.7150/ijms.8522
Gruhn B, Naumann T, Gruner D, Walther M, Wittig S, Becker S, Beck JF, Sonnemann J (2013) The expression of histone deacetylase 4 is associated with prednisone poor-response in childhood acute lymphoblastic leukemia. Leuk Res 37(10):1200–1207. doi:10.1016/j.leukres.2013.07.016
New M, Olzscha H, La Thangue NB (2012) HDAC inhibitor-based therapies: can we interpret the code? Mol Oncol 6(6):637–656. doi:10.1016/j.molonc.2012.09.003
Seto E, Yoshida M (2014) Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol 6(4):a018713. doi:10.1101/cshperspect.a018713
Heltweg B, Gatbonton T, Schuler AD, Posakony J, Li H, Goehle S, Kollipara R, Depinho RA, Gu Y, Simon JA, Bedalov A (2006) Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res 66(8):4368–4377. doi:10.1158/0008-5472.CAN-05-3617
Ropero S, Esteller M (2007) The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 1(1):19–25. doi:10.1016/j.molonc.2007.01.001
Glozak MA, Seto E (2009) Acetylation/deacetylation modulates the stability of DNA replication licensing factor Cdt1. J Biol Chem 284(17):11446–11453. doi:10.1074/jbc.M809394200
Witt O, Deubzer HE, Lodrini M, Milde T, Oehme I (2009) Targeting histone deacetylases in neuroblastoma. Curr Pharm Des 15(4):436–447
Caron C, Boyault C, Khochbin S (2005) Regulatory cross-talk between lysine acetylation and ubiquitination: role in the control of protein stability. Bioessays 27(4):408–415. doi:10.1002/bies.20210
Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325(5942):834–840. doi:10.1126/science.1175371
Bereshchenko OR, Gu W, Dalla-Favera R (2002) Acetylation inactivates the transcriptional repressor BCL6. Nat Genet 32(4):606–613. doi:10.1038/ng1018
Gu W, Roeder RG (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90(4):595–606
Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, Horinouchi S, Yoshida M (2002) In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 21(24):6820–6831
Grignani F, De Matteis S, Nervi C, Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara FF, Zamir I, Seiser C, Lazar MA, Minucci S, Pelicci PG (1998) Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 391(6669):815–818. doi:10.1038/35901
Cress WD, Seto E (2000) Histone deacetylases, transcriptional control, and cancer. J Cell Physiol 184(1):1–16. doi:10.1002/(SICI)1097-4652(200007)184:1<1::AID-JCP1>3.0.CO;2–7
Yang H, Maddipoti S, Quesada A, Bohannan Z, Cabrero Calvo M, Colla S, Wei Y, Estecio M, Wierda W, Bueso-Ramos C, Garcia-Manero G (2015) Analysis of class I and II histone deacetylase gene expression in human leukemia. Leuk Lymphoma 26:1–8. doi:10.3109/10428194.2015.1034705
Chun P (2015) Histone deacetylase inhibitors in hematological malignancies and solid tumors. Arch Pharm Res 38(6):933–949. doi:10.1007/s12272-015-0571-1
Riggs MG, Whittaker RG, Neumann JR, Ingram VM (1977) n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature 268(5619):462–464
Grant S, Easley C, Kirkpatrick P (2007) Vorinostat. Nat Rev Drug Discov 6(1):21–22. doi:10.1038/nrd2227
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5(9):769–784. doi:10.1038/nrd2133
Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind RA, Marks PA (1998) A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci U S A 95(6):3003–3007
Kirschbaum M, Frankel P, Popplewell L, Zain J, Delioukina M, Pullarkat V, Matsuoka D, Pulone B, Rotter AJ, Espinoza-Delgado I, Nademanee A, Forman SJ, Gandara D, Newman E (2011) Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol 29(9):1198–1203. doi:10.1200/JCO.2010.32.1398
Ogura M, Ando K, Suzuki T, Ishizawa K, Oh SY, Itoh K, Yamamoto K, Au WY, Tien HF, Matsuno Y, Terauchi T, Mori M, Tanaka Y, Shimamoto T, Tobinai K, Kim WS (2014) A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol 165(6):768–776. doi:10.1111/bjh.12819
Nakajima H, Kim YB, Terano H, Yoshida M, Horinouchi S (1998) FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp Cell Res 241(1):126–133. doi:10.1006/excr.1998.4027
Byrd JC, Marcucci G, Parthun MR, Xiao JJ, Klisovic RB, Moran M, Lin TS, Liu S, Sklenar AR, Davis ME, Lucas DM, Fischer B, Shank R, Tejaswi SL, Binkley P, Wright J, Chan KK, Grever MR (2005) A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood 105(3):959–967. doi:10.1182/blood-2004-05-1693
Klimek VM, Fircanis S, Maslak P, Guernah I, Baum M, Wu N, Panageas K, Wright JJ, Pandolfi PP, Nimer SD (2008) Tolerability, pharmacodynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodysplastic syndromes. Clin Cancer Res 14(3):826–832. doi:10.1158/1078-0432.CCR-07-0318
Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, Zain J, Prince HM, Leonard JP, Geskin LJ, Reeder C, Joske D, Figg WD, Gardner ER, Steinberg SM, Jaffe ES, Stetler-Stevenson M, Lade S, Fojo AT, Bates SE (2009) Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol 27(32):5410–5417. doi:10.1200/JCO.2008.21.6150
Bates SE, Zhan Z, Steadman K, Obrzut T, Luchenko V, Frye R, Robey RW, Turner M, Gardner ER, Figg WD, Steinberg SM, Ling A, Fojo T, To KW, Piekarz RL (2010) Laboratory correlates for a phase II trial of romidepsin in cutaneous and peripheral T-cell lymphoma. Br J Haematol 148(2):256–267. doi:10.1111/j.1365-2141.2009.07954.x
Gimsing P (2009) Belinostat: a new broad acting antineoplastic histone deacetylase inhibitor. Expert Opin Investig Drugs 18(4):501–508. doi:10.1517/13543780902852560
Zain JM, O’Connor O (2010) Targeted treatment and new agents in peripheral T-cell lymphoma. Int J Hematol 92(1):33–44. doi:10.1007/s12185-010-0614-9
Foss F, Advani R, Duvic M, Hymes KB, Intragumtornchai T, Lekhakula A, Shpilberg O, Lerner A, Belt RJ, Jacobsen ED, Laurent G, Ben-Yehuda D, Beylot-Barry M, Hillen U, Knoblauch P, Bhat G, Chawla S, Allen LF, Pohlman B (2015) A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol 168(6):811–819. doi:10.1111/bjh.13222
De Souza C, Chatterji BP (2015) HDAC inhibitors as novel anti-cancer therapeutics. Recent Pat Anticancer Drug Discov 10(2):145–162
Gong K, Xie J, Yi H, Li W (2012) CS055 (Chidamide/HBI-8000), a novel histone deacetylase inhibitor, induces G1 arrest, ROS-dependent apoptosis and differentiation in human leukaemia cells. Biochem J 443(3):735–746. doi:10.1042/BJ20111685
Dong M, Ning ZQ, Xing PY, Xu JL, Cao HX, Dou GF, Meng ZY, Shi YK, Lu XP, Feng FY (2012) Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother Pharmacol 69(6):1413–1422. doi:10.1007/s00280-012-1847-5
Rambaldi A, Dellacasa CM, Finazzi G, Carobbio A, Ferrari ML, Guglielmelli P, Gattoni E, Salmoiraghi S, Finazzi MC, Di Tollo S, D’Urzo C, Vannucchi AM, Barosi G, Barbui T (2010) A pilot study of the histone-deacetylase inhibitor Givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol 150(4):446–455. doi:10.1111/j.1365-2141.2010.08266.x
Duvic M, Dummer R, Becker JC, Poulalhon N, Ortiz Romero P, Grazia Bernengo M, Lebbe C, Assaf C, Squier M, Williams D, Marshood M, Tai F, Prince HM (2013) Panobinostat activity in both bexarotene-exposed and -naive patients with refractory cutaneous T-cell lymphoma: results of a phase II trial. Eur J Cancer 49(2):386–394. doi:10.1016/j.ejca.2012.08.017
Dickinson M, Ritchie D, DeAngelo DJ, Spencer A, Ottmann OG, Fischer T, Bhalla KN, Liu A, Parker K, Scott JW, Bishton M, Prince HM (2009) Preliminary evidence of disease response to the pan deacetylase inhibitor panobinostat (LBH589) in refractory Hodgkin lymphoma. Br J Haematol 147(1):97–101. doi:10.1111/j.1365-2141.2009.07837.x
DeAngelo DJ, Spencer A, Bhalla KN, Prince HM, Fischer T, Kindler T, Giles FJ, Scott JW, Parker K, Liu A, Woo M, Atadja P, Mishra KK, Ottmann OG (2013) Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia 27(8):1628–1636. doi:10.1038/leu.2013.38
Rao R, Balusu R, Fiskus W, Mudunuru U, Venkannagari S, Chauhan L, Smith JE, Hembruff SL, Ha K, Atadja P, Bhalla KN (2012) Combination of pan-histone deacetylase inhibitor and autophagy inhibitor exerts superior efficacy against triple-negative human breast cancer cells. Mol Cancer Ther 11(4):973–983. doi:10.1158/1535-7163.MCT-11-0979
Chateauvieux S, Morceau F, Dicato M, Diederich M (2010) Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol. doi:10.1155/2010/479364
Bokelmann I, Mahlknecht U (2008) Valproic acid sensitizes chronic lymphocytic leukemia cells to apoptosis and restores the balance between pro- and antiapoptotic proteins. Mol Med 14(1–2):20–27. doi:10.2119/2007-00084.Bokelmann
Saito A, Yamashita T, Mariko Y, Nosaka Y, Tsuchiya K, Ando T, Suzuki T, Tsuruo T, Nakanishi O (1999) A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc Natl Acad Sci U S A 96(8):4592–4597
Pili R, Salumbides B, Zhao M, Altiok S, Qian D, Zwiebel J, Carducci MA, Rudek MA (2012) Phase I study of the histone deacetylase inhibitor entinostat in combination with 13-cis retinoic acid in patients with solid tumours. Br J Cancer 106(1):77–84. doi:10.1038/bjc.2011.527
Bonfils C, Kalita A, Dubay M, Siu LL, Carducci MA, Reid G, Martell RE, Besterman JM, Li Z (2008) Evaluation of the pharmacodynamic effects of MGCD0103 from preclinical models to human using a novel HDAC enzyme assay. Clin Cancer Res 14(11):3441–3449. doi:10.1158/1078-0432.CCR-07-4427
Boumber Y, Younes A, Garcia-Manero G (2011) Mocetinostat (MGCD0103): a review of an isotype-specific histone deacetylase inhibitor. Expert Opin Investig Drugs 20(6):823–829. doi:10.1517/13543784.2011.577737
Siu LL, Pili R, Duran I, Messersmith WA, Chen EX, Sullivan R, MacLean M, King S, Brown S, Reid GK, Li Z, Kalita AM, Laille EJ, Besterman JM, Martell RE, Carducci MA (2008) Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. J Clin Oncol 26(12):1940–1947. doi:10.1200/JCO.2007.14.5730
Rivera-Del Valle N, Gao S, Miller CP, Fulbright J, Gonzales C, Sirisawad M, Steggerda S, Wheler J, Balasubramanian S, Chandra J (2010) PCI-24781, a novel hydroxamic acid HDAC inhibitor, exerts cytotoxicity and histone alterations via caspase-8 and FADD in leukemia cells. Int J Cell Biol 2010:207420. doi:10.1155/2010/207420
Morschhauser F, Terriou L, Coiffier B, Bachy E, Varga A, Kloos I, Lelievre H, Sarry AL, Depil S, Ribrag V (2015) Phase 1 study of the oral histone deacetylase inhibitor abexinostat in patients with Hodgkin lymphoma, non-Hodgkin lymphoma, or chronic lymphocytic leukaemia. Investig New Drugs 33(2):423–431. doi:10.1007/s10637-015-0206-x
Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL (2003) Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A 100(8):4389–4394. doi:10.1073/pnas.0430973100
Balasubramanian S, Ramos J, Luo W, Sirisawad M, Verner E, Buggy JJ (2008) A novel histone deacetylase 8 (HDAC8)-specific inhibitor PCI-34051 induces apoptosis in T-cell lymphomas. Leukemia 22(5):1026–1034. doi:10.1038/leu.2008.9
Methot JL, Chakravarty PK, Chenard M, Close J, Cruz JC, Dahlberg WK, Fleming J, Hamblett CL, Hamill JE, Harrington P, Harsch A, Heidebrecht R, Hughes B, Jung J, Kenific CM, Kral AM, Meinke PT, Middleton RE, Ozerova N, Sloman DL, Stanton MG, Szewczak AA, Tyagarajan S, Witter DJ, Secrist JP, Miller TA (2008) Exploration of the internal cavity of histone deacetylase (HDAC) with selective HDAC1/HDAC2 inhibitors (SHI-1:2). Bioorg Med Chem Lett 18(3):973–978. doi:10.1016/j.bmcl.2007.12.031
Thurn KT, Thomas S, Moore A, Munster PN (2011) Rational therapeutic combinations with histone deacetylase inhibitors for the treatment of cancer. Future Oncol 7(2):263–283. doi:10.2217/fon.11.2
Li L, Wang L, Wang Z, Ho Y, McDonald T, Holyoake TL, Chen W, Bhatia R (2012) Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell 21(2):266–281. doi:10.1016/j.ccr.2011.12.020
Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, Altucci L, Nervi C, Minucci S, Pelicci PG (2005) Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med 11(1):71–76. doi:10.1038/nm1160
Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM (1982) Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A 79(24):7824–7827
Taub R, Kirsch I, Morton C, Lenoir G, Swan D, Tronick S, Aaronson S, Leder P (1982) Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A 79(24):7837–7841
Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, Korsmeyer SJ (1985) Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell 41(3):899–906
Cleary ML, Sklar J (1985) Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A 82(21):7439–7443
Liu H, Wang J, Epner EM (2004) Cyclin D1 activation in B-cell malignancy: association with changes in histone acetylation, DNA methylation, and RNA polymerase II binding to both promoter and distal sequences. Blood 104(8):2505–2513. doi:10.1182/blood-2004-02-0483
Singh BN, Zhang G, Hwa YL, Li J, Dowdy SC, Jiang S-W (2010) Nonhistone protein acetylation as cancer therapy targets. Expert Rev Anticancer Ther 10(6):935–954. doi:10.1586/era.10.62
Glozak MA, Sengupta N, Zhang X, Seto E (2005) Acetylation and deacetylation of non-histone proteins. Gene 363:15–23. doi:10.1016/j.gene.2005.09.010
Dokmanovic M, Clarke C, Marks PA (2007) Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5(10):981–989. doi:10.1158/1541-7786.MCR-07-0324
Eot-Houllier G, Fulcrand G, Magnaghi-Jaulin L, Jaulin C (2009) Histone deacetylase inhibitors and genomic instability. Cancer Lett 274(2):169–176. doi:10.1016/j.canlet.2008.06.005
Grant C, Rahman F, Piekarz R, Peer C, Frye R, Robey RW, Gardner ER, Figg WD, Bates SE (2010) Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther 10(7):997–1008. doi:10.1586/era.10.88
Cotto M, Cabanillas F, Tirado M, Garcia MV, Pacheco E (2010) Epigenetic therapy of lymphoma using histone deacetylase inhibitors. Clin Transl Oncol 12(6):401–409. doi:10.1007/s12094-010-0527-3
Batova A, Shao LE, Diccianni MB, Yu AL, Tanaka T, Rephaeli A, Nudelman A, Yu J (2002) The histone deacetylase inhibitor AN-9 has selective toxicity to acute leukemia and drug-resistant primary leukemia and cancer cell lines. Blood 100(9):3319–3324. doi:10.1182/blood-2002-02-0567
Cortez CC, Jones PA (2008) Chromatin, cancer and drug therapies. Mutat Res 647(1–2):44–51. doi:10.1016/j.mrfmmm.2008.07.006
Garcia-Manero G, Assouline S, Cortes J, Estrov Z, Kantarjian H, Yang H, Newsome WM, Miller WH Jr, Rousseau C, Kalita A, Bonfils C, Dubay M, Patterson TA, Li Z, Besterman JM, Reid G, Laille E, Martell RE, Minden M (2008) Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood 112(4):981–989. doi:10.1182/blood-2007-10-115873
Jin Y, Cao Q, Chen C, Du X, Jin B, Pan J (2015) Tenovin-6-mediated inhibition of SIRT1/2 induces apoptosis in acute lymphoblastic leukemia (ALL) cells and eliminates ALL stem/progenitor cells. BMC Cancer 15:226. doi:10.1186/s12885-015-1282-1
Liu HL, Chen Y, Cui GH, Zhou JF (2005) Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation. Acta Pharmacol Sin 26(5):603–609. doi:10.1111/j.1745-7254.2005.00081.x
Acknowledgments
This work was supported by grants from the Institut National de Cancer (ERABL) and the Laboratoires Servier (PHA7854 014).
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markozashvili, D., Ribrag, V. & Vassetzky, Y.S. Histone deacetylase inhibitors and epigenetic regulation in lymphoid malignancies. Invest New Drugs 33, 1280–1291 (2015). https://doi.org/10.1007/s10637-015-0290-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0290-y