Summary
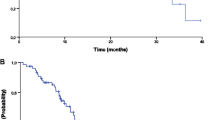
Background Phase I trials of the microtubule stabilising agent patupilone showed encouraging tumour control and response rates in patients with metastatic colorectal cancer. Methods Patients with metastatic or locally recurrent colorectal cancer who had progressed following treatment with oxaliplatin, irinotecan and fluoropyrimidines were treated with patupilone (8 mg/m2 IV every 3 weeks) in combination with dexamethasone or prednisolone. Results The trial was closed early after 29 patients had been enrolled due to concerns about toxicity. 20 patients (71.4 %) experienced at least one grade 3–5 toxicity, most commonly diarrhoea (14 patients), dehydration (7 patients) and lethargy (6 patients). The 12 week progression-free survival rate was 16.7 % (95 % CI 6.1 %–36.5 %) in the 24 patients with a 12 week scan available or who had died prior to the 12 week scan. No complete or partial responses were seen by 12 weeks. The median progression-free survival was 2.6 months (95 % CI 2.3–2.9) and median overall survival was 6.1 months (95 % CI 3.7–8.4). Conclusion Patupilone given at a dose of 8 mg/m2 IV over 20 min every 3 weeks was associated with high levels of toxicity and no significant evidence of efficacy in patients with pre-treated colorectal cancer.


Similar content being viewed by others
References
Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N (2010) Colorectal cancer. Lancet 375(9719):1030–1047. doi:https://doi.org/10.1016/S0140-6736(10)60353-4
Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P (2007) Estimates of the cancer incidence and mortality in Europe in 2006. Annal Oncol Off J Eur Soc Med Oncol / ESMO 18(3):581–592. doi:https://doi.org/10.1093/annonc/mdl498
Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D (2009) Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer 9(7):489–499. doi:https://doi.org/10.1038/nrc2645
Swanton C, Tomlinson I, Downward J (2006) Chromosomal instability, colorectal cancer and taxane resistance. Cell cycle 5(8):818–823
Bystricky B, Chau I (2011) Patupilone in cancer treatment. Expert Opin Investigational Drugs 20(1):107–117. doi:https://doi.org/10.1517/13543784.2011.542148
Anand S, Penrhyn-Lowe S, Venkitaraman AR (2003) AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer cell 3(1):51–62
Melichar B, Casado E, Bridgewater J, Bennouna J, Campone M, Vitek P, Delord JP, Cerman J Jr, Salazar R, Dvorak J, Sguotti C, Urban P, Viraswami-Appanna K, Tan E, Tabernero J (2011) Clinical activity of patupilone in patients with pretreated advanced/metastatic colon cancer: results of a phase I dose escalation trial. Br J Cancer 105(11):1646–1653. doi:https://doi.org/10.1038/bjc.2011.438
Iqbal S, El-Khoueiry AB, Yang D, Cole S, Boswell W, Shriki J, Ning Y, Agafitei RD, Menendez X, Lenz H (2010) A phase I study of celecoxib (C) and patupilone (EPO906) in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol Off J Am S Clin Oncol 28 (suppl; abstr 2533)
McSheehy P, Becquet M, Boisclair J, Bizot MN (2008) Prednisolone abrogates patupilone (EPO906)-induced diarrhoea in rats without impacting on patupilone PK or efficacy. Proc 20th EORTC-NCI-AACR Symp Mol Targets Cancer Ther EJC Suppl 6:451
Sridhar SS, Hotte SJ, Kollmannsberger CK, Mukherjee SD, Capier K, Barclay J, Adams L, Weber D, Chi KN (2010) Preventing patupilone-induced diarrhea with high-dose corticosteroids. J Clin Oncol Off J Am S Clin Oncol 28 (suppl; abstr e13069)
Kornblau S, Benson AB, Catalano R, Champlin RE, Engelking C, Field M, Ippoliti C, Lazarus HM, Mitchell E, Rubin J, Stiff PJ, Vokes E, Wadler S (2000) Management of cancer treatment-related diarrhea. Issues and therapeutic strategies. J Pain Symptom Manag 19(2):118–129
Wadler S, Benson AB 3rd, Engelking C, Catalano R, Field M, Kornblau SM, Mitchell E, Rubin J, Trotta P, Vokes E (1998) Recommended guidelines for the treatment of chemotherapy-induced diarrhea. J Clin Oncol Off J Am S Clin Oncol 16(9):3169–3178
Poplin E, Moore M, O'Dwyer P, Clarke S, Hill M, Sessa C, Rothermel J, Mull R, Miller J, L; R (2003) Safety and efficacy of EPO906 in patients with advanced colorectal cancer: A review of 2 phase II trials Proc Am Soc Clin Oncol 22 (abstr 1135)
Colombo N, Kutarska E, Dimopoulos M, Bae DS, Rzepka-Gorska I, Bidzinski M, Scambia G, Engelholm SA, Joly F, Weber D, El-Hashimy M, Li J, Souami F, Wing P, Engelholm S, Bamias A, Schwartz P (2012) Randomized, open-label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. J Clin Oncol Off J Am S Clin Oncol 30(31):3841–3847. doi:https://doi.org/10.1200/JCO.2011.38.8082
Hussain A, DiPaola RS, Baron AD, Higano CS, Tchekmedyian NS, Johri AR (2009) Phase II trial of weekly patupilone in patients with castration-resistant prostate cancer. Annal Oncol Off J Eur Soc Med Oncol / ESMO 20(3):492–497. doi:https://doi.org/10.1093/annonc/mdn665
Reid TR, Takimoto CH, Verschraegen CF, Sarantopoulos J, Cheung W, Allen-Freda E, Li J, Xu Y, Ko J, Johri A (2008) Evaluation of safety, tolerability and pharmacokinetics (PK) of patupilone in patients (pts) with advanced solid tumors and varying degrees of hepatic function: An open-label phase I study. J Clin Oncol Off J Am S Clin Oncol 26: (May 20 suppl; abstr 2557)
Chi KN, Beardsley E, Eigl BJ, Venner P, Hotte SJ, Winquist E, Ko YJ, Sridhar SS, Weber D, Saad F (2012) A phase 2 study of patupilone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel: Canadian Urologic Oncology Group study P07a. Annal Oncol Off J Eur Soc Med Oncol / ESMO 23(1):53–58. doi:https://doi.org/10.1093/annonc/mdr336
Abrey LE, Wen P, Govindan R, Reimers H, Rigas JR, Robins HI, Allen-Freda E, Gao B, Ko J, Johri A (2008) Patupilone for the treatment of recurrent/progressive brain metastases in patients (pts) with non-small cell lung cancer (NSCLC): An open-label phase II study. J Clin Oncol Off J Am S Clin Oncol 26: (May 20 suppl; abstr 2033)
De Souza PL, Mellado B, Pfister C, Rosenthal M, Castellano DE, Weber D, Ferrara S, Shaik N, Tan E, S.G; P (2010) Randomized phase II trial of patupilone plus prednisone versus docetaxel plus prednisone in patients with chemotherapy-naïve, metastatic, castrate-resistant prostate cancer (CRPC). J Clin Oncol Off J Am S Clin Oncol 28 (suppl; abstr 4553)
Oehler C, Frei K, Rushing EJ, McSheehy PM, Weber D, Allegrini PR, Weniger D, Lutolf UM, Knuth A, Yonekawa Y, Barath K, Broggini-Tenzer A, Pruschy M, Hofer S (2012) Patupilone (epothilone B) for recurrent glioblastoma: clinical outcome and translational analysis of a single-institution phase I/II trial. Oncology 83(1):1–9. doi:https://doi.org/10.1159/000339152
Conlin AK, D'Andrea G, Hudis CA, Robson ME, Drullinsky P, Theodoulou M, Lis E, Kang TY, Peereboom DM, Seidman AD (2008) Phase II trial of patupilone in patients (pts) with breast cancer brain metastases (BCBM) progressing or recurring after whole brain radiation therapy (WBXRT) Journal of Clinical Oncology 26 (May 20 Suppl;1086)
Rubin EH, Rothermel J, Tesfaye F, Chen T, Hubert M, Ho YY, Hsu CH, Oza AM (2005) Phase I dose-finding study of weekly single-agent patupilone in patients with advanced solid tumors. J Clin Oncol Off J Am S Clin Oncol 23(36):9120–9129. doi:https://doi.org/10.1200/JCO.2005.03.0981
Acknowledgements
We acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre and Novartis who provided an educational grant and patupilone.
Ethical standards
The study was approved by a Research Ethics Committee and all patients provided written informed consent.
Conflicts of interest
IC has received research funding from Novartis, Merck-Serono and Roche, and has advisory roles (compensated) with Roche, Sanofi-Aventis, Novartis and Eli Lilly. DC has received research funding from Amgen, Roche, Sanofi-Aventis, Merck-Serono, Novartis, and Celgene, and has had advisory roles (uncompensated) with Roche and Amgen. SR has received research funding from GlaxoSmithKline and has advisory roles (uncompensated) with Roche, Sanofi-Aventis, Merck-Serono and Celgene. CS sat on a global advisory board for Novartis 3 years ago and receives research funding from Novartis. SYM, CP, KLY, AW, CJ, GS, JW, GSm and AG have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. L. Yim, A. Walther and C. Jackson were working at The Royal Marsden at the time of this research.
Rights and permissions
About this article
Cite this article
Moorcraft, S.Y., Chau, I., Peckitt, C. et al. Patupilone in patients with pretreated metastatic/locally recurrent colorectal cancer: results of the Phase II CINATRA trial. Invest New Drugs 31, 1339–1344 (2013). https://doi.org/10.1007/s10637-013-9990-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-9990-3