Summary
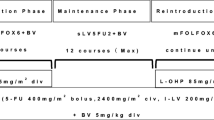
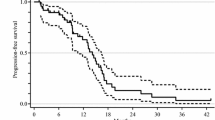
Currently, no prospective data exists to support a “stop-and-go” modified FOLFOX6 regimen with bevacizumab in metastatic colorectal cancer (mCRC) patients. This study aimed to evaluate the efficacy and safety of this regimen in first-line mCRC patients. Eligible patients (age ≥20 years) had previously untreated mCRC; Eastern Cooperative Oncology Group performance status of 0–2; and adequate hematologic, hepatic, and renal function. The modified FOLFOX6 regimen and bevacizumab (5 mg/kg) was administered intravenously every 2 weeks. After 8 cycles, patients received maintenance therapy with simplified LV5FU2 and bevacizumab until completion of 8 cycles or disease progression. After maintenance therapy, patients received another 8 cycles of modified FOLFOX6 with bevacizumab until completion of 8 cycles or disease progression. We recruited 50 patients between August 2007 and January 2009. The overall response rate was 48% (80% confidence interval [CI]; 38.2–58) with outcomes as follows: complete response, n = 1; partial response, n = 23; stable disease, n = 21; progression, n = 1; and not evaluated, n = 4. Median time to treatment failure was 7.7 months (80% CI: 6.2–8.0), and median progression-free survival was 12.8 months (80% CI: 10.8–14). Grade 3/4 toxicities included neutropenia (40%), nausea (4%), diarrhea (14%), thrombosis (4%), and hypertension (4%) et al. Grade 1, 2, or 3 peripheral neuropathy was reported in 38%, 40%, and 10% of patients, respectively. The stop-and-go modified FOLFOX6 and bevacizumab regimen is effective and well tolerated as first-line chemotherapy for mCRC patients.


Similar content being viewed by others
References
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Giantonio BJ, Catalano PJ, Meropol NJ et al (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544
Saltz LB, Clarke S, Diaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019
Goldberg RM, Sargent DJ, Morton RF et al (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30
Andre T, Boni C, Boudiaf LM et al (2004) Oxaliplatin, fluorouracil and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
Grothey A, Nikcevich DA, Sloan JA et al (2011) Intravenous calcium and magnesium for oxaliplatin-induced sensory neurotoxicity in adjuvant colon cancer: NCCTG N04C7. J Clin Oncol 29:421–427
Saif MW, Syrigos K, Kaley K, Isufi (2010) Role of pregabalin in treatment of oxaliplatin-induced sensory neuropathy. Anticancer Res 30:2927–2934
Tournigand C, Cervantes A, Figer A et al (2006) OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer-a GERCOR study. J Clin Oncol 24:394–400
Grothey A, Hart LL, Rowland KM, et al. (2008) Intermittent oxaliplatin (oxali) administration and time-to-treatment-failure (TTF) in metastatic colorectal cancer (mCRC): Final results of the phase III CONcePT trial. J Clin Oncol 26S: Abstr 4010.
Hochster HS, Hart LL, Ramanathan RK et al (2008) Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE study. J Clin Oncol 26:3523–3529
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
National Cancer Institute-Common Toxicity Criteria. (NCI-CTC Version 3.0, March 31, 2003).
Kato K, Inaba Y, Tsuji Y et al (2011) A multicenter phase-II study of 5-FU, leucovorin and oxaliplatin (FOLFOX6) in patients with pretreated metastatic colorectal cancer. Jpn J Clin Oncol 41:63–68
Yasui H, Hamaguchi T, Shimada Y, et al. (2008) A multicenter phase-II study of 5-FU, leucovorin and oxaliplatin (FOLFOX6) in patients with previously untreated metastatic colorectal cancer. The 4th Annual Meeting of Japanese Society of Clinical Oncology P-183
Kalbfleisch JD, Prentice RL (2002) The statistical analysis of failure time data, 2nd edn. Wiley, Hoboken
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Controlled Clinical Trials 17:343–346
Chibaudel B, Maindrault-Goebel F, Gerard Lled et al (2009) Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 study. J Clin Oncol 27:5727–5733
Vaidyanathan G, Groman A, Wilding G, Fakih MG (2010) Stop and go FOLFOX plus bevacizumab chemotherapy in the first-line treatment of metastatic colorectal cancer. Oncology 79:67–71
Hochster HS, Grothey A, Shpilsky A, Childs BH (2008) Effect of intravenous calcium and magnesium versus placebo on response to FOLFOX + bevacizumab in the CONcePT trial. J Clin Oncol (ASCO-GI 2008 Abstract No.280)
de Gramont A, Figer A, Seymour M et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Acknowledgements
We thank Hiroshi Yoshida, Aasako Sakamoto and Makiko Shinogi for data collection, and Yushi Nagai and Michiyo Tada for data management. This work was supported by Grants-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan [grant number 20S-3].
Conflict of interest statements
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okita, N.T., Esaki, T., Baba, E. et al. A multicenter phase II study of the stop-and-go modified FOLFOX6 with bevacizumab for first-line treatment of patients with metastatic colorectal cancer. Invest New Drugs 30, 2026–2031 (2012). https://doi.org/10.1007/s10637-011-9779-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9779-1