Summary
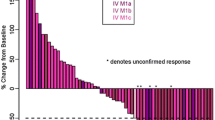
Objective: Ipilimumab is a fully human, anti–cytotoxic T-lymphocyte antigen-4 (CTLA-4) monoclonal antibody that has demonstrated antitumor activity in advanced melanoma. We evaluated the safety and efficacy of ipilimumab alone and in combination with dacarbazine (DTIC) in patients with unresectable, metastatic melanoma. Methods: Chemotherapy-naïve patients were randomized in this multicenter, phase II study to receive ipilimumab at 3 mg/kg every 4 weeks for four doses either alone or with up to six 5-day courses of DTIC at 250 mg/m2/day. The primary efficacy endpoint was objective response rate. Results: Seventy-two patients were treated per-protocol (ipilimumab plus DTIC, n = 35; ipilimumab, n = 37). The objective response rate was 14.3% (95% CI, 4.8–30.3) with ipilimumab plus DTIC and was 5.4% (95% CI, 0.7–18.2) with ipilimumab alone. At a median follow-up of 20.9 and 16.4 months for ipilimumab plus DTIC (n = 32) and ipilimumab alone (n = 32), respectively, median overall survival was 14.3 months (95% CI, 10.2–18.8) and 11.4 months (95% CI, 6.1–15.6); 12-month, 24-month, and 36-month survival rates were 62%, 24% and 20% for the ipilimumab plus DTIC group and were 45%, 21% and 9% for the ipilimumab alone group, respectively. Immune-related adverse events were, in general, medically manageable and occurred in 65.7% of patients in the combination group versus 53.8% in the monotherapy group, with 17.1% and 7.7% ≥grade 3, respectively. Conclusion: Ipilimumab therapy resulted in clinically meaningful responses in advanced melanoma patients, and the results support further investigations of ipilimumab in combination with DTIC.


Similar content being viewed by others
References
Lens MB, Dawes M (2004) Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol 150:179–185
Cancer Facts & Figures (2009) American cancer society, Atlanta, GA, USA. http://www.cancer.org/downloads/STT/2009CAFFfinalsecured.pdf Accessed August 31st, 2009
Korn EL, Liu PY, Lee SJ et al (2008) Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 26:527–534
Bedikian AY, Millward M, Pehamberger H et al (2006) Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the oblimersen melanoma study group. J Clin Oncol 24:4738–4745
Agarwala SS (2009) Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther 9:587–595
Petrella T, Quirt I, Verma S, Haynes AE, Charette M, Bak K, Melanoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care (2007) Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat Rev 33:484–496
Peggs KS, Segal NH, Allison JP (2007) Targeting immunosupportive cancer therapies: accentuate the positive, eliminate the negative. Cancer Cell 12:192–199
Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L (2007) Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer 7:95–106
Morse MA (2005) Technology evaluation: Ipilimumab, Medarex/Bristol-Myers Squibb. Curr Opin Mol Ther 7:588–597
Cranmer LD, Hersh E (2007) The role of the CTLA4 blockade in the treatment of malignant melanoma. Cancer Invest 25:613–631
Fong L, Small EJ (2008) Anti–cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol 26:5275–5283
Ridolfi L, Ridolfi R (2009) Anti-CTLA-4 therapy in melanoma: role of ipilimumab (MDX-010). Expert Rev Dermatol 4:199–210
Hodi FS, Mihm MC, Soiffer RJ et al (2003) Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA 100:4712–4717
Langer LF, Clay TM, Morse MA (2007) Update on anti-CTLA-4 antibodies in clinical trials. Expert Opin Biol Ther 7:1245–1256
Margolin K (2008) Moving forward with immunotherapy: the rationale for anti-CTLA-4 therapy in melanoma. Comm Oncol 5:367–374
O’Day SJ, Ibrahim R, DePril V et al (2008) Efficacy and safety of ipilimumab induction and maintenance dosing in patients with advanced melanoma who progressed on one or more prior therapies. J Clin Oncol 26(19s):abstract 9021
Weber JS, O’Day S, Urba W et al (2008) Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 26:5950–5956
Phan GQ, Yang JC, Sherry RM et al (2003) Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 100:8372–8377
Attia P, Phan GQ, Maker AV et al (2005) Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 23:6043–6053
Maker AV, Phan GQ, Attia P et al (2005) Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol 12:1005–1016
Wolchok JD, Neyns B, Linette G et al (2009) Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol; in press
Urba WJ, Weber JS, O’Day SJ et al (2008) Long-term survival of patients with advanced melanoma who received ipilimumab administered at 10mg/kg every 3weeks for 4 doses (induction dosing). J Clin Oncol 26(19s):abstract 3018
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Lake RA, Robinson BW (2005) Immunotherapy and chemotherapy–a practical partnership. Nat Rev Cancer 5:397–405
Zigler M, Villares GJ, Lev DC, Melnikova VO, Bar-Eli M (2008) Tumor immunotherapy in melanoma: strategies for overcoming mechanisms of resistance and escape. Am J Clin Dermatol 9:307–311
Ribas A, Camacho LH, Lopez Berestein G et al (2005) Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675, 206. J Clin Oncol 23:8968–8977
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Weber J (2009) Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother 58:823–830
Lin R, Yellin MJ, Lowy I, Safferman A, Chin K, Ibrahim R (2008) An analysis of the effectiveness of specific guidelines for the management of ipilimumab-mediated diarrhea/colitis: prevention of gastrointestinal perforation and/or colectomy. J Clin Oncol 26(19s):abstract 9063
Weber J (2007) Review: Anti–CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist 12:864–872
Weber J, Thompson JA, Hamid O et al (2009) A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 15:5591–5598
ClinicalTrials.gov. Dacarbazine and ipilimumab vs. dacarbazine with placebo in untreated, unresectable stage III or IV melanoma. www.clinicaltrials.gov/ct/show/NCT00324155 Accessed September 3rd, 2009
Acknowledgments
The authors thank Steven A. Fischkoff (currently at Palatin Technologies, Cranbury, NJ) for technical assistance and Georgia Brockway for assistance in preparing and reviewing the data. Writing and editorial support was provided by StemScientific, funded by Bristol-Myers Squibb Co.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hersh, E.M., O’Day, S.J., Powderly, J. et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs 29, 489–498 (2011). https://doi.org/10.1007/s10637-009-9376-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9376-8