Abstract
Purpose
Usher syndrome (USH) is a multisensory deficiency involving vision, hearing and the vestibular system. The purpose of this study is to report on the functional data (i.e. electroretinography, visual fields, visual acuity) of patients with retinitis pigmentosa (RP) due to Usher syndrome that were collected in a multicentre European study (TREATRUSH).
Methods
A total of 268 genetically confirmed USH patients underwent electrophysiological examinations in the context of multimodal ophthalmological examination in the study (75 USH1, 189 USH2 and four USH3). Full-field electroretinography (ERG) was performed according to ISCEV standards, visual field determination was carried out with either the Octopus or Goldmann perimeters and visual acuity was examined with either ETDRS or Snellen charts. The data were compared between USH subtypes (USH1/USH2/USH3) and correlated with age.
Results
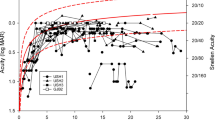
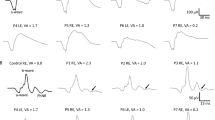
Visual acuity decreases significantly with age for both USH1 and USH2 (p < 0.001), without a difference between the two cohorts. When corrected for age, the preserved kinetic visual field was significantly larger in USH2 than in USH1 (p = 0.04). Furthermore, the preserved kinetic visual field area showed a significant decrease with age (based on an exponential fit) in both USH1 and USH2 (p < 0.001). In USH1 patients, however, the visual field was already vastly reduced at an early age. The ERG results were abnormal in all patients. Detectable data for scotopic ERG were obtained from nine patients, and data of photopic ERG were obtained from 24 patients, without a difference between USH1 and USH2 subtypes.
Conclusions
There are differences in the phenotypes of RP in USH subtypes, most visible in the progression of visual fields between USH1 and USH2. The perimetric reduction occurs earlier in USH1 than in USH2. In both subtypes, visual acuity decreases significantly with age and the ERG is not detectable already at early ages.




Similar content being viewed by others
References
Zrada SE, Braat K, Doty RL, Laties AM (1996) Olfactory loss in Usher syndrome: Another sensory deficit? Am J Med Genet 64(4):602–603
Petit C (2001) Usher syndrome: from genetics to pathogenesis. Annu Rev Genom Hum Genet 2:271–297
Fakin A, Jarc-Vidmar M, Glavač D, Bonnet C, Petit C, Hawlina M (2012) Fundus autofluorescence and optical coherence tomography in relation to visual function in Usher syndrome type 1 and 2. Vision Res 15(75):60–70
Pakarinen L, Tuppurainen K, Laippala P, Mäntyjärvi M, Puhakka H (1995–1996) The ophthalmological course of Usher syndrome type III. Int Ophthalmol 19(5):307–11
Ness SL, Ben-Yosef T, Bar-Lev A, Madeo AC, Brewer CC, Avraham KB et al (2003) Genetic homogeneity and phenotypic variability among Ashkenazi Jews with Usher syndrome type III. J Med Genet 40(10):767–772
Bonnet C, Riahi Z, Chantot-Bastaraud S, Smagghe L, Letexier M, Marcaillou C et al (2016) An innovative strategy for the molecular diagnosis of Usher syndrome identifies causal biallelic mutations in 93% of European patients. Eur J Hum Genet EJHG 24(12):1730–1738
Iannaccone A, Kritchevsky SB, Ciccarelli ML, Tedesco SA, Macaluso C, Kimberling WJ et al (2004) Kinetics of visual field loss in Usher syndrome Type II. Invest Ophthalmol Vis Sci 45(3):784–792
Pierrache LHM, Hartel BP, van Wijk E, Meester-Smoor MA, Cremers FPM, de Baere E et al (2016) Visual prognosis in USH2A-associated retinitis pigmentosa is worse for patients with usher syndrome type IIa than for those with nonsyndromic retinitis pigmentosa. Ophthalmology 123(5):1151–1160
Sengillo JD, Cabral T, Schuerch K, Duong J, Lee W, Boudreault K et al (2017) Electroretinography reveals difference in cone function between syndromic and nonsyndromic USH2A patients. Sci Rep 7(1):11170
Lenassi E, Saihan Z, Cipriani V, Le Quesne Stabej P, Moore AT, Luxon LM et al (2014) Natural history and retinal structure in patients with Usher syndrome type 1 owing to MYO7A mutation. Ophthalmology 121(2):580–587
Fleischhauer J, Njoh WA, Niemeyer G (2005) Syndromic retinitis pigmentosa: ERG and phenotypic changes. Klin Monatsbl Augenheilkd 222(3):186–190
Malm E, Ponjavic V, Möller C, Kimberling WJ, Stone ES, Andréasson S (2011) Alteration of rod and cone function in children with Usher syndrome. Eur J Ophthalmol 21(1):30–38
Mets MB, Young NM, Pass A, Lasky JB (2000) Early diagnosis of Usher syndrome in children. Trans Am Ophthalmol Soc 98:237–242 discussion 243-245
Sandberg MA, Rosner B, Weigel-DiFranco C, McGee TL, Dryja TP, Berson EL (2008) Disease course in patients with autosomal recessive retinitis pigmentosa due to the USH2A gene. Invest Ophthalmol Vis Sci 49(12):5532–5539
Tsilou ET, Rubin BI, Caruso RC, Reed GF, Pikus A, Hejtmancik JF et al (2002) Usher syndrome clinical types I and II: Could ocular symptoms and signs differentiate between the two types? Acta Ophthalmol Scand 80(2):196–201
Edwards A, Fishman GA, Anderson RJ, Grover S, Derlacki DJ (1998) Visual acuity and visual field impairment in Usher syndrome. Arch Ophthalmol 116(2):165–168
Testa F, Melillo P, Bonnet C, Marcelli V, de Benedictis A, Colucci R et al (2017) Clinical presentation and disease course of usher syndrome because of mutations in MYO7A OR USH2A. c Phila PA 37(8):1581–1590
Pennings RJE, Huygen PLM, Orten DJ, Wagenaar M, van Aarem A, Kremer H et al (2004) Evaluation of visual impairment in Usher syndrome 1b and Usher syndrome 2a. Acta Ophthalmol Scand 82(2):131–139
Nagy D, Schönfisch B, Zrenner E, Jägle H (2008) Long-term follow-up of retinitis pigmentosa patients with multifocal electroretinography. Invest Ophthalmol Vis Sci 49(10):4664–4671
Mendieta L, Berezovsky A, Salomão SR, Sacai PY, Pereira JM, Fantini SC (2005) Visual acuity and full-field electroretinography in patients with Usher’s syndrome. Arq Bras Oftalmol 68(2):171–176
Zein WM, Falsini B, Tsilou ET, Turriff AE, Schultz JM, Friedman TB et al (2015) Cone responses in Usher syndrome types 1 and 2 by microvolt electroretinography. Invest Ophthalmol Vis Sci 56(1):107–114
Sieving PA, Arnold EB, Jamison J, Liepa A, Coats C (1998) Submicrovolt flicker electroretinogram: cycle-by-cycle recording of multiple harmonics with statistical estimation of measurement uncertainty. Invest Ophthalmol Vis Sci 39(8):1462–1469
Iannaccone A (2003) Usher syndrome: correlation between visual field size and maximal ERG response b-wave amplitude. Adv Exp Med Biol 533:123–131
Sahly I, Dufour E, Schietroma C, Michel V, Bahloul A, Perfettini I et al (2012) Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. J Cell Biol 199(2):381–399
Schietroma C, Parain K, Estivalet A, Aghaie A, Boutet de Monvel J, Picaud S et al (2017) Usher syndrome type 1-associated cadherins shape the photoreceptor outer segment. J Cell Biol 216(6):1849–1864
Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC et al (2007) Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci U S A 104(11):4413–4418
Maerker T, van Wijk E, Overlack N, Kersten FFJ, McGee J, Goldmann T et al (2008) A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum Mol Genet 17(1):71–86
Yang J, Liu X, Zhao Y, Adamian M, Pawlyk B, Sun X et al (2010) Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genet 6(5):1000955
Kaiser PK (2009) Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). Trans Am Ophthalmol Soc 107:311–324
Funding
This work was supported by the European Union Seventh Framework Programme under the Grant Agreement HEALTH-F2-2010-242013 (TREATRUSH) and the Tistou and Charlotte Kerstan Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership or other equity interest; and expert testimony or patent licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stingl, K., Kurtenbach, A., Hahn, G. et al. Full-field electroretinography, visual acuity and visual fields in Usher syndrome: a multicentre European study. Doc Ophthalmol 139, 151–160 (2019). https://doi.org/10.1007/s10633-019-09704-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-019-09704-8