Abstract
Aims
This study aimed to explore the protection mechanism of ISO-1 on severe acute pancreatitis-associated intrahepatic bile duct (IBD) injury in rats.
Methods
Forty-eight specific-pathogen-free male Wistar rats were randomly divided into four groups (N = 12): a sham operation group (SO group), a severe acute pancreatitis model group (SAP group), a ISO-1 treatment group (ISO-1 + SAP group), and a ISO-1 control group (ISO-1 + SO group). All rats were killed after 12 h of being made models. Immunohistochemistry was used to detect the expression of MIF and P38 in IBD cells. MIF mRNA expression in IBD cells was observed using real-time fluorescent quantitative polymerase chain reaction (real-time PCR). In addition, Western blotting was performed to detect the protein expression of P38, phosphorylated P38 (P-P38), nuclear factor-κB (NF-κB p65), and tumor necrosis factor alpha (TNF-α). Enzyme-linked immunosorbent assays were used to analyze the levels of TNF-α, IL-1β, and IL-6 in the IBD of rats.
Results
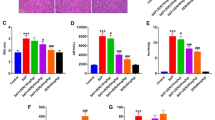
Compared with SAP, after treatment with ISO-1, the pathological injuries of pancreas, liver, and IBD cells in ISO-1 treatment group remarkably relieved. The expression of MIF in the IBD cells was significantly downregulated both at mRNA and at protein levels in ISO-1 treatment group. Besides, the protein expression levels of P38, P-P38, NF-κBp65, TNF-α, IL-1β, and IL-6 in the IBD in rats were also significantly decreased in ISO-1 treatment group (all P < 0.05).
Conclusion
ISO-1 may protect the IBD cells, reduce pathological injuries, and reduce the inflammatory response in SAP rats. Its mechanisms may be via inhibiting the expression of MIF and then blocking the activation of p38-MAPK and NF-κB signaling pathway.










Similar content being viewed by others
Abbreviations
- MIF:
-
Macrophage migration inhibitory factor
- ISO-1:
-
A specific small molecule inhibitor of MIF
- SAP:
-
Severe acute pancreatitis
- AP:
-
Acute pancreatitis
- IBD:
-
Intrahepatic bile duct
- SPF:
-
Specific-pathogen-free
- SO:
-
Sham operation
- NF-κB:
-
Nuclear factor-κB
- TNF:
-
Tumor necrosis factor
- ELISA:
-
Enzyme-linked immunosorbent assays
- IL:
-
Interleukin
- MODS:
-
Multiple organ dysfunction syndrome
- MOF:
-
Multiple organ failure
- SIRS:
-
Systemic inflammatory response syndrome
- ARDS:
-
Acute respiratory distress syndrome
- AP:
-
Acute pancreatitis
- MAPK:
-
Mitogen-activated protein kinase
- ICAM:
-
Intercellular adhesion molecule
- VCAM:
-
Vascular cell adhesion protein
- MCP:
-
Monocyte chemoattractant protein
- HRP:
-
Horseradish peroxidase
- H&E:
-
Hematoxylin and eosin
- PBS:
-
Phosphate-buffered saline
- IOD:
-
Integral optical density
- SEM:
-
Standard error of the mean
References
Wang B, Zhao KL, Hu WJ, Zuo T, Ding YM, Wang WX. Macrophage migration inhibitor promoted the intrahepatic bile duct injury in rats with severe acute pancreatitis. Dig Dis Sci. 2019;64:759–772. https://doi.org/10.1007/s10620-018-5379-7.
Aeberli D, Yang Y, Mansell A, et al. Endogenous macrophage migration inhibitory factor modulates glucocorticoid sensitivity in macrophages via effects on MAP kinase phosphatase-1 and p38 MAP kinase. FEBS Lett. 2006;580:974–981.
Chuang CC, Chuang YC, Chang WT, et al. Macrophage migration inhibitory factor regulates interleukin-6 production by facilitating nuclear factor-κB activation during Vibrio vulnificus infection. BMC Immunol. 2010;11:50.
Takahashi K, Koga K, Linge HM, et al. Macrophage CD74 contributes to MIF-induced pulmonary inflammation. Respir Res. 2009;10:33.
Paszt A, Eder K, Szabolcs A, et al. Effects of glucocorticoid agonist and antagonist on the pathogenesis of l-arginine-induced acute pancreatitis in rat. Pancreas. 2008;36:369–376.
Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56.
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, et al. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993,5:265–1272.
Lubetsky JB, Dios A, Han J, et al. The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J Biol Chem. 2002;277:24976–24982.
Sakai Y, Masamune A, Satoh A, et al. Macrophage migration inhibitory factor is a critical mediator of severe acute pancreatitis. Gastroenterology. 2003;124:725–736.
Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800.
Mazzon E, Impellizzeri D, Di Paola R, et al. Effects of mitogen-activated protein kinase signaling pathway inhibition on the development of cerulein-induced acute pancreatitis in mice. Pancreas. 2012;41:560–570.
Nattee P, Honsawek S, Chongsrisawat V, et al. Elevated serum macrophage migration inhibitory factor levels in post-operative biliary atresia. Asian J Surg. 2009;32:109–113.
Nakajima H, Takagi H, Horiguchi N, et al. Lack of macrophage migration inhibitory factor protects mice against concanavalin A-induced liver injury. Liver Int. 2006;26:346–351.
Chuang CC, Wang ST, Chen WC, et al. Increases in serum macrophage migration inhibitory factor in patients with severe sepsis predict early mortality. Shock. 2007;27:503–506.
Murr MM, Yang J, Fier A, et al. Regulation of Kupffer cell TNF gene expression during experimental acute pancreatitis: the role of p38-MAPK, ERK1/2, SAPK/JNK, and NF-κB. J Gastrointest Surg. 2003;7:20–25.
Murr MM, Yang J, Fier A, et al. Pancreatic elastase induces liver injury by activating cytokine production within Kupffer cells via nuclear factor-κB. J Gastrointest Surg. 2002;6:474–480.
Hietaranta A, Mustonen H, Puolakkainen P, et al. Proinflammatory effects of pancreatic elastase are mediated through TLR4 and NF-κB. Biochem Biophys Res Commun. 2004;323:192–196.
Cuzzocrea S, Mazzon E, Di Paola R, et al. Glycogen synthase kinase-3 beta inhibition attenuates the degree of arthritis caused by type II collagen in the mouse. Clin Immunol. 2006;120:57–67.
Yu J, Deng WH, Wang WX, et al. Inhibition of poly(ADP-ribose) polymerase attenuates acute kidney injury in sodium taurocholate-induced acute pancreatitis in rats. Pancreas. 2012;41:1299–1305.
Deng H, Zhang N, Wang Y, et al. S632A3, a new glutarimide antibiotic, suppresses lipopolysaccharide-induced pro-inflammatory responses via inhibiting the activation of glycogen synthase kinase 3 beta. Exp Cell Res. 2012;318:2592–2603.
Deng WH, YuJ, Wang WX, et al. Zerumbone attenuates the severity of acute necrotizing pancreatitis and pancreatitis-induced hepatic injury. Mediators Inflamm, 2012, 2012: 156507.
Schafer C, Tietz AB, Goke B. Pathophysiology of acute experimental pancreatitis: lessons from genetically engineered animal models and new molecular approaches. Digestion. 2005;71:162–172.
Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24:45–51.
Pastor CM, Frossard JL. Are genetically modified mice useful for the understanding of acute pancreatitis. FASEB J. 2001;15:893–897.
Gulcubuk A, Altunatmaz K, Sonmez K, et al. Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. J Vet Med A Physiol Pathol Clin Med. 2006;53:49–54.
Abcouwer S F, Norman J, Fink G, et al. Tissue-specific regulation of glutamine synthetase gene expression in acute pancreatitis is confirmed by using interleukin-1 receptor knockout mice. Surgery, 1996, 120: 255–263, 263–264.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study has no conflict of interest with any organization or individual.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, B., Zhao, K., Hu, W. et al. Protective Mechanism of MIF Inhibitor ISO-1 on Intrahepatic Bile Duct Cells in Rats with Severe Acute Pancreatitis. Dig Dis Sci 66, 3415–3426 (2021). https://doi.org/10.1007/s10620-020-06674-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06674-9