Abstract
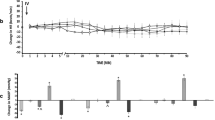
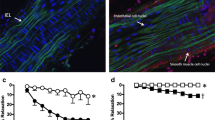
Hydrogen sulfide (H2S) is a recently discerned endogenous signaling molecule that modulates the vascular system. Endogenous hydrogen sulfide has been shown to dilate both the mesenteric and portal vasculature. Gut microbiome, via sulfur reducing bacteria, is another source of H2S production within the gut lumen; this source of H2S is primarily produced and detoxified in the colon under physiologic conditions. Nitric oxide (NO), a major endogenous vasodilator in the portal circulation, participates in H2S-induced vasodilation in some vascular beds. We hypothesize that jejunal but not colonic H2S increases portal vein flow in a NO-dependent fashion. To evaluate the effects of luminal H2S, venous blood flow, portal venous pressure, and systemic venous pressure were measured in rats after administration of either vehicle or an H2S donor (NaHS) into the jejunum or the colon. We found that portal venous pressure and systemic pressure did not change and were similar between the three study groups. However, portal venous blood flow significantly increased following jejunal administration of NaHS but not in response to colonic NaHS or vehicle administration. To test the contribution of NO production to this response, another group of animals was treated with either an NO synthase inhibitor (N-Ω-nitro-l-arginine, L-NNA) or saline prior to jejunal NaHS infusion. After L-NNA pretreatment, NaHS caused a significant fall rather than increase in portal venous flow compared to saline pretreatment. These data demonstrate that H2S within the small intestine significantly increases portal venous blood flow in a NO-dependent fashion.


Similar content being viewed by others
References
Kanagy NL, Szabo C, Papapetropoulos A. Vascular biology of hydrogen sulfide. Am J Physiol Cell Physiol. 2017;312:C537–C549.
Naik JS, Osmond JM, Walker BR, Kanagy NL. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Am J Physiol Hear Circ Physiol. 2016;311:H1437–H1444.
Mani S, Cao W, Wu L, Wang R. Hydrogen sulfide and the liver. Nitric Oxide Biol Chem. 2014;41:62–71.
Norris EJ, Feilen N, Nguyen NH, et al. Hydrogen sulfide modulates sinusoidal constriction and contributes to hepatic micorcirculatory dysfunction during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1070–G1078.
Fiorucci S, Antonelli E, Mencarelli A, et al. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548.
Feng X, Zhang H, Shi M, Chen Y, Yang T, Fan H. Toxic effects of hydrogen sulfide donor NaHS induced liver apoptosis is regulated by complex IV subunits and reactive oxygen species generation in rats. Environ Toxicol. 2020;35:322–332.
Wallace JL, Ferraz JGP, Muscara MN. Hydrogen sulfide: an endogenous mediator of resolution of inflammation and injury. Antioxidants Redox Signal. 2012;17:58–67.
Nakamura N, Lin HC, McSweeney CS, Mackie RI, Gaskins HR. Mechanisms of microbial hydrogen disposal in the human colon and implications for health and disease. Annu Rev Food Sci Technol. 2010;1:363–395.
Barton LL, Ritz NL, Fauque GD, Lin HC. Sulfur cycling and the intestinal microbiome. Dig Dis Sci. 2017;62:2241–2257. https://doi.org/10.1007/s10620-017-4689-5.
Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J Clin Investig. 1999;104:1107–1114.
Beaumont M, Andriamihaja M, Lan A, et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: the adaptive response. Free Radic Biol Med. 2016;93:155–164.
Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem Pharmacol. 2001;62:255–259.
Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis: current status and future directions. J Hepatol. 2014;61:912–924.
Gaynullina DK, Sofronova SI, Selivanova EK, et al. NO-mediated anticontractile effect of the endothelium is abolished in coronary arteries of adult rats with antenatal/early postnatal hypothyroidism. Nitric Oxide Biol Chem. 2017;63:21–28.
Suarez F, Furne J, Springfield J, Levitt M. Production and elimination of sulfur-containing gases in the rat colon. Am J Physiol. 1998;274:727–733.
Lin HC. Small intestinal bacterial overgrowth. JAMA. 2004;292:852.
Hee Kang S, Young Kim M, Koo Baik S. SPECIAL ISSUE-PORTAL HYPERTENSION Novelties in the pathophysiology and management of portal hypertension: new treatments on the horizon. Hepatol Int. 2012;12:112–121.
Tumgor G. Cirrhosis and hepatopulmonary syndrome. World J Gastroenterol. 2014;20:2586–2594.
Arroyo V, Ginés P. Arteriolar vasodilation and the pathogenesis of the hyperdynamic circulation and renal sodium and water retention in cirrhosis. Gastroenterology. 1992;102:1077–1079.
Møller S, Bendtsen F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018;38:570–580.
Bolognesi M, Di Pascoli M, Verardo A, Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol. 2014;20:2555–2563.
Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Dig Dis. 2016;34:382–386.
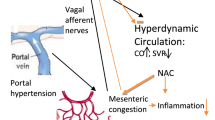
Huc T, Jurkowska H, Wróbel M, Jaworska K, Onyszkiewicz M, Ufnal M. Colonic hydrogen sulfide produces portal hypertension and systemic hypotension in rats. Exp Biol Med. 2018;243:96–106.
Matallo J, Vogt J, Mccook O, et al. Sulfide-inhibition of mitochondrial respiration at very low oxygen concentrations. Nitric Oxide Biol Chem. 2014;41:79–84.
Hildebrandt MA, Hoffman C, Sherrill-Mix SA, et al. High fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009. https://doi.org/10.1053/j.gastro.2009.08.042.
Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544–545.
Mackos AR, Maltz R, Bailey MT. The role of the commensal microbiota in adaptive and maladaptive stressor-induced immunomodulation. Horm Behav. 2017;88:70–78.
Chassard C, Dapoigny M, Scott KP, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828–838.
Norris EJ, Culberson CR, Narasimhan S, Clemens MG. The liver as a central regulator of hydrogen sulfide. Shock. 2011;36:242–250.
Henao-Mejia J, Elinav E, Thaiss CA, Licona-Limon P, Flavell RA. Role of the intestinal microbiome in liver disease. J Autoimmun. 2013;46:66–73.
Da Silva HE, Teterina A, Comelli EM, et al. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-19753-9.
Xie G, Wang X, Liu P, Wei R, Chen W, Rajani C, et al. Distinctly altered gut microbiota in the progression of liver disease. 2016; [cited 2020 May 4]. www.impactjournals.com/oncotarget.
Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433.
Runyon BA, Squier S, Borzio M. Translocation of gut bacteria in rats with cirrhosis to mesenteric lymph nodes partially explains the pathogenesis of spontaneous bacterial peritonitis. J Hepatol. 1994;21:792–796.
Guarner C, González-Navajas JM, Sánchez E, et al. The detection of bacterial DNA in blood of rats with CCl4-induced cirrhosis with ascites represents episodes of bacterial translocation. Hepatology. 2006;44:633–639.
García-Pagán JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458–461.
Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18:281–291.
Acharya C, Bajaj JS. Accepted manuscript the microbiome in cirrhosis and its complications. Clin Gastroenterol Hepatol. 2018. https://doi.org/10.1016/j.cgh.2018.08.008.
Albillos A, Bañares R, González M, et al. The extent of the collateral circulation influences the postprandial increase in portal pressure in patients with cirrhosis. Gut. 2007;56:259–264.
Ginés P, Quintero E, Arroyo V, et al. Compensated cirrhosis: Natural history and prognostic factors. Hepatology. 1987;7:122–128.
Acknowledgments
This study is supported, in part, by: University of New Mexico Research Allocation Grant and HL123301. Dr. Lin was supported, in part, by the Winkler Bacterial Overgrowth Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have related IP rights.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Birg, A., Lin, H.C. & Kanagy, N. Portal Venous Flow Is Increased by Jejunal but Not Colonic Hydrogen Sulfide in a Nitric Oxide-Dependent Fashion in Rats. Dig Dis Sci 66, 2661–2668 (2021). https://doi.org/10.1007/s10620-020-06597-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06597-5