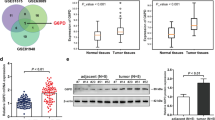
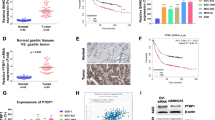
Abstract
Background
Gastric cancer (GC) is one of the most commonly diagnosed malignancy worldwide. DLX6 antisense RNA 1 (DLX6-AS1) is a long noncoding RNA (lncRNA) that exhibits oncogenic effects on multiple human carcinomas.
Aims
This study aimed to investigate the regulatory effect of DLX6-AS1 in GC progression.
Methods
The expression of DLX6-AS1 in GC tissues and cell lines was examined. The cell viability, number of clones, and apoptosis, aerobic glycolysis, and mitochondrial respiration was assessed. The effect of DLX6-AS1 on tumor growth in nude mice was also evaluated.
Results
DLX6-AS1 was overexpressed in GC tissues and cell lines. DLX6-AS1 knockdown by short hairpin RNA (shRNA) significantly inhibited cell viability and colony formation, and induced apoptosis. DLX6-AS1 silencing impaired aerobic glycolysis but stimulated mitochondrial respiration in GC cells. miR-4290 was confirmed as a downstream target of DLX6-AS1, and their expression levels were inversely correlated. GC cells expressing sh-DLX6-AS1 showed significantly lower level of 3-phosphoinositide-dependent protein kinase 1 (PDK1), a target of miR-4290, compared to cells expressing control shRNA. In addition, the suppressed GC cell malignancy upon DLX6-AS1 knockdown could be prominently reversed by PDK1 overexpression. Meanwhile, PDK1 overexpression enhanced aerobic glycolysis but repressed mitochondrial respiration under sh-DLX6-AS1 treatment. Furthermore, DLX6-AS1 knockdown significantly delayed the tumor growth in a mouse xenograft model inoculated with GC cells.
Conclusions
LncRNA DLX6-AS1 regulated tumor growth and aerobic glycolysis in GC by targeting miR-4290 and PDK1, suggesting DLX6-AS1 might serve as a novel potential therapeutic target for GC treatment from bench to clinic.






Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26–38. https://doi.org/10.5114/pg.2018.80001.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep. 2017;19:36. https://doi.org/10.1007/s11894-017-0575-8.
Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007). Gastric Cancer Offic J Int Gastr Cancer Assoc Jpn Gastr Cancer Assoc. 2018;21:144–154. https://doi.org/10.1007/s10120-017-0716-7.
Chen LL, Carmichael GG. Long noncoding RNAs in mammalian cells: what, where, and why? Wiley Interdiscip Rev RNA. 2010;1:2–21. https://doi.org/10.1002/wrna.5.
Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. https://doi.org/10.1038/nm.3981.
Hao NB, He YF, Li XQ, Wang K, Wang RL. The role of miRNA and lncRNA in gastric cancer. Oncotarget. 2017;8:81572–81582. https://doi.org/10.18632/oncotarget.19197.
Chen JF, Wu P, Xia R, et al. STAT3-induced lncRNA HAGLROS overexpression contributes to the malignant progression of gastric cancer cells via mTOR signal-mediated inhibition of autophagy. Mol Cancer. 2018;17:6. https://doi.org/10.1186/s12943-017-0756-y.
Zhao J, Du P, Cui P, et al. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018;37:4094–4109. https://doi.org/10.1038/s41388-018-0250-z.
Zhao Y, Liu Y, Lin L, et al. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol Cancer. 2018;17:69. https://doi.org/10.1186/s12943-018-0820-2.
Zeng X, Hu Z, Ke X, et al. Long noncoding RNA DLX6-AS1 promotes renal cell carcinoma progression via miR-26a/PTEN axis. Cell Cycle (Georgetown, Tex). 2017;16:2212–2219. https://doi.org/10.1080/15384101.2017.1361072.
Zhang L, He X, Jin T, Gang L, Jin Z. Long non-coding RNA DLX6-AS1 aggravates hepatocellular carcinoma carcinogenesis by modulating miR-203a/MMP-2 pathway. Biomed Pharmacother Biomed Pharmacother. 2017;96:884–891. https://doi.org/10.1016/j.biopha.2017.10.056.
Li J, Li P, Zhao W, et al. Expression of long non-coding RNA DLX6-AS1 in lung adenocarcinoma. Cancer Cell Int. 2015;15:48. https://doi.org/10.1186/s12935-015-0201-5.
Fu X, Tian Y, Kuang W, Wen S, Guo W. Long non-coding RNA DLX6-AS1 silencing inhibits malignant phenotypes of gastric cancer cells. Exp Ther Med. 2019;17:4715–4722. https://doi.org/10.3892/etm.2019.7521.
Salehi Z, Akrami H, Sisakhtnezhad S. The effect of placenta growth factor knockdown on hsa-miR-22-3p, hsa-let-7b-3p, hsa-miR-451b, and hsa-mir-4290 expressions in MKN-45-derived gastric cancer stem-like cells. Middle East J Cancer. 2018;9:113–122.
Zhang C, Zhang CD, Ma MH, Dai DQ. Three-microRNA signature identified by bioinformatics analysis predicts prognosis of gastric cancer patients. World J Gastroenterol. 2018;24:1206–1215. https://doi.org/10.3748/wjg.v24.i11.1206.
Zhang SL, Hu X, Zhang W, Tam KY. Unexpected discovery of dichloroacetate derived adenosine triphosphate competitors targeting pyruvate dehydrogenase kinase to inhibit cancer proliferation. J Med Chem. 2016;59:3562–3568. https://doi.org/10.1021/acs.jmedchem.5b01828.
Dupuy F, Tabaries S, Andrzejewski S, et al. PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 2015;22:577–589. https://doi.org/10.1016/j.cmet.2015.08.007.
Peng F, Wang JH, Fan WJ, et al. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene. 2018;37:1062–1074. https://doi.org/10.1038/onc.2017.368.
World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. https://doi.org/10.1001/jama.2013.281053.
Zhu Z, Friess H, Kleeff J, et al. Glypican-3 expression is markedly decreased in human gastric cancer but not in esophageal cancer. Am J Surg. 2002;184:78–83. https://doi.org/10.1016/s0002-9610(02)00884-x.
Hong X, Xu Y, Qiu X, et al. MiR-448 promotes glycolytic metabolism of gastric cancer by downregulating KDM2B. Oncotarget. 2016;7:22092–22102. https://doi.org/10.18632/oncotarget.8020.
National Research Council Institute for Laboratory Animal R. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US) Copyright 1996 by the National Academy of Sciences. All rights reserved; 1996.
Alenzi FQ. Links between apoptosis, proliferation and the cell cycle. Br J Biomed Sci. 2004;61:99–102.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. https://doi.org/10.1038/nrc1478.
Justus CR, Sanderlin EJ, Yang LV. Molecular connections between cancer cell metabolism and the tumor microenvironment. Int J Mol Sci. 2015;16:11055–11086. https://doi.org/10.3390/ijms160511055.
Beitzinger M, Peters L, Zhu JY, Kremmer E, Meister G. Identification of human microRNA targets from isolated argonaute protein complexes. RNA Biol. 2007;4:76–84. https://doi.org/10.4161/rna.4.2.4640.
Lin MT, Song HJ, Ding XY. Long non-coding RNAs involved in metastasis of gastric cancer. World J Gastroenterol. 2018;24:3724–3737. https://doi.org/10.3748/wjg.v24.i33.3724.
Gao S, Zhao ZY, Wu R, Zhang Y, Zhang ZY. Prognostic value of long noncoding RNAs in gastric cancer: a meta-analysis. OncoTargets Therapy. 2018;11:4877–4891. https://doi.org/10.2147/ott.s169823.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY).. 2009;324:1029–1033. https://doi.org/10.1126/science.1160809.
Ngo DC, Ververis K, Tortorella SM, Karagiannis TC. Introduction to the molecular basis of cancer metabolism and the Warburg effect. Mol Biol Rep. 2015;42:819–823. https://doi.org/10.1007/s11033-015-3857-y.
Yuan LW, Yamashita H, Seto Y. Glucose metabolism in gastric cancer: the cutting-edge. World J Gastroenterol. 2016;22:2046–2059. https://doi.org/10.3748/wjg.v22.i6.2046.
Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34:9–14. https://doi.org/10.1016/j.semcdb.2014.05.015.
Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA interactions. Methods Mol Biol (Clifton, NJ). 2016;1402:271–286. https://doi.org/10.1007/978-1-4939-3378-5_21.
Castel P, Ellis H, Bago R, et al. PDK1-SGK1 signaling sustains AKT-independent mTORC1 activation and confers resistance to PI3Kalpha inhibition. Cancer Cell. 2016;30:229–242. https://doi.org/10.1016/j.ccell.2016.06.004.
Eser S, Reiff N, Messer M, et al. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell. 2013;23:406–420. https://doi.org/10.1016/j.ccr.2013.01.023.
Wu YH, Chang TH, Huang YF, Chen CC, Chou CY. COL11A1 confers chemoresistance on ovarian cancer cells through the activation of Akt/c/EBPbeta pathway and PDK1 stabilization. Oncotarget. 2015;6:23748–23763. https://doi.org/10.18632/oncotarget.4250.
Zhang L, Lei J, Fang ZL, Xiong JP. MiR-128b is down-regulated in gastric cancer and negatively regulates tumour cell viability by targeting PDK1/Akt/NF-kappaB axis. J Biosci. 2016;41:77–85.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YQ and TFX conceived and designed the experiments, WS and XW analyzed and interpreted the results of the experiments, GWH, HXW and XH performed the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest, and all authors should confirm its accuracy.
Human and animal rights statement
All experimental protocols were approved by the Ethics Committee of the Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University and performed following the World Medical Association Declaration of Helsinki. All animal experimental procedures were approved by the Animal Care and Use Committee of the Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University and were performed under the Guide for the Care and Use of Laboratory Animals.
Informed consent
Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article. All the patients signed written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qian, Y., Song, W., Wu, X. et al. DLX6 Antisense RNA 1 Modulates Glucose Metabolism and Cell Growth in Gastric Cancer by Targeting microRNA-4290. Dig Dis Sci 66, 460–473 (2021). https://doi.org/10.1007/s10620-020-06223-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06223-4