Abstract
Background
Golimumab is a new anti-TNF-alpha monoclonal antibody for patients with ulcerative colitis.
Aims
To assess the short- and long-term effectiveness and safety of golimumab in daily clinical practice and to identify predictors of response.
Methods
Consecutive patients treated with golimumab in 22 Italian centers were enrolled. Clinical, laboratory, and endoscopic data were prospectively collected before and during treatment. A subgroup of patients completed a questionnaire to assess personal satisfaction with a golimumab autoinjector system.
Results
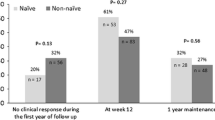
A total of 196 patients were included. After 3 months, 130 patients were responders (66.3%) and showed significant reductions in mean partial, total, and endoscopic Mayo scores and in mean ESR, C-reactive protein, and fecal calprotectin levels (p < 0.001). Multivariate analysis revealed that a higher total Mayo score (p < 0.001, OR 1.5, 95% CI 1.2–1.8) and naïve status to anti-TNF-alpha (p = 0.015, OR 3.0, 95% CI 1.2–7.5) were predictive of a favorable response. Seventy-seven (39.3%) of the 130 responders maintained a response at month 12 of therapy. There were 17 adverse events, 28 patients needed hospitalization, and 15 patients underwent surgery. Self-administration of the drug was appreciated by most patients.
Conclusions
The efficacy and safety of golimumab in daily clinical practice were confirmed for the short- and long-term treatment of patients with active ulcerative colitis. Patients naïve to the anti-TNF-alpha monoclonal antibody and those with a higher total Mayo score were more likely to respond to golimumab.




Similar content being viewed by others
References
Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on the diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2018;11:769–784.
Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on the diagnosis and management of ulcerative colitis. Part 1: definition, diagnosis, extraintestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;6:649–670.
D’Haens G, Panaccione R, Higgins P, et al. The London position statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212.
Hutas G. Golimumab as the first monthly subcutaneous fully human anti-TNF-alpha antibody in the treatment of inflammatory arthropathies. Immunotherapy. 2010;2(4):453–60.
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95.
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate to severe ulcerative colitis. Gastroenterology. 2014;146:96–109.
Blonde L, Khunti K, Harris SB. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–1774.
Castro-Laria L, Argüelles-Arias F, García-Sánchez V, et al. Initial experience with golimumab in clinical practice for ulcerative colitis. Rev Esp Enferm Dig. 2016;108:129–132.
Bosca Watts MM, Cortes X, Iborra M, et al. Short-term effectiveness of golimumab for ulcerative colitis: observational multicenter study. World J Gastroenterol. 2016;22:10432–10439.
Tursi A, Allegretta L, Buccianti N, et al. Effectiveness and safety of golimumab in treating outpatient ulcerative colitis: a real-life prospective, multicentre, observational study in primary inflammatory bowel diseases centers. J Gastrointestin Liver Dis. 2017;26:239–244.
Taxonera C, Rodríguez C, Bertoletti F. Clinical outcomes of golimumab as first, second or third anti-TNF agent in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2017;23:1394–1402.
Probert CS, Sebastian S, Gaya DR, et al. Golimumab induction and maintenance for moderate to severe ulcerative colitis: results from GO-COLITIS (Golimumab: a Phase 4, UK, open label, single arm study on its utilization and impact in ulcerative colitis). BMJ Open Gastroenterol. 2018;5:e000212.
Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6.
Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:5A–36A.
Schroeder KW, Tremaine WJ, Ilstrup DM, et al. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. New Engl J Med. 1987;317:1625–1629.
Orlando A, Armuzzi A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1–20.
Likert R. A technique for the measurement of attitude. Arch Psychol. 1932;140:1–55.
Schulze-Koops H, Giacomelli R, Samborski W, et al. Patient evaluations of autoinjectors for delivery of subcutaneous golimumab for treatment of rheumatoid arthritis. Ann Rheum Dis. 2013;72:A230–A231.
Tandorn N, Bolce R, Naim A, et al. Satisfaction with and preference for golimumab and its auto-injector among rheumatoid arthritis patients switched from adalimumab or etanercept. Value Health. 2012;15:A45–A46.
Funding
None.
Author information
Authors and Affiliations
Contributions
FB: planning the study, drafting the article, analysis and interpretation of data. AA, GB, FWG, AR, MBP, MC, AT, WF, ACP, GC, LG, and SM: planning the study, critical revision of the article and interpretation of data. MRV: analysis of data. AL, RC, CF, ES, MM, GP, LS, AM, MP, RS, CR, CS, PP, GI, AAz, ON, NB, MR, GR, LF, and RM: critical revision of the article for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bossa, F., Biscaglia, G., Valvano, M.R. et al. Real-Life Effectiveness and Safety of Golimumab and Its Predictors of Response in Patients with Ulcerative Colitis. Dig Dis Sci 65, 1767–1776 (2020). https://doi.org/10.1007/s10620-019-05904-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05904-z