Abstract
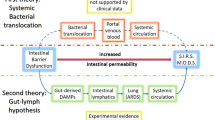
Sepsis is a life-threatening response to systemic infection. In addition to frank gastrointestinal (GI) rupture/puncture, sepsis can also be exacerbated by translocation of pathogen-associated molecular patterns (PAMPs) from the GI tract to the systemic circulation (gut origin of sepsis). In the human gut, Gram-negative bacteria and Candida albicans are abundant, along with their major PAMP components, endotoxin (LPS) and (1 → 3)-β-d-glucan (BG). Whereas the influence of LPS in bacterial sepsis has been studied extensively, exploration of the role of BG in bacterial sepsis is limited. Post-translocation, PAMPs enter the circulation through lymphatics and the portal vein, and are detoxified and then excreted via the liver and the kidney. Sepsis-induced liver and kidney injury might therefore affect the kinetics and increase circulating PAMPs. In this article, we discuss the current knowledge of the impact of PAMPs from both gut mycobiota and microbiota, including epithelial barrier function and the “gut-liver-kidney axis,” on bacterial sepsis severity.


Similar content being viewed by others
References
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–810.
Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
Bates JM, Akerlund J, Mittge E, et al. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382.
Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818.
Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268.
Baranova IN, Vishnyakova TG, Bocharov AV, et al. Class B scavenger receptor types I and II and CD36 mediate bacterial recognition and proinflammatory signaling induced by Escherichia coli, lipopolysaccharide, and cytosolic chaperonin 60. J Immunol. 2012;188:1371–1380.
Doi K, Hu X, Yuen PS, et al. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73:1266–1274.
Doi K, Leelahavanichkul A, Yuen PS, et al. Animal models of sepsis and sepsis-induced kidney injury. J Clin Investig. 2009;119:2868–2878.
Leelahavanichkul A, Bocharov AV, Kurlander R, et al. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J Immunol. 2012;188:2749–2758.
Leelahavanichkul A, Huang Y, Hu X, et al. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int. 2011;80:1198–1211.
Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, et al. Gastrointestinal leakage detected by serum (1 ⟶ 3)-beta-d-glucan in mouse models and a pilot study in patients with sepsis. Shock. 2016;46:506–518.
Leelahavanichkul A, Yasuda H, Doi K, et al. Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am J Physiol Renal Physiol. 2008;295:F1825–F1835.
Panpetch W, Somboonna N, Bulan DE, et al. Gastrointestinal colonization of Candida albicans increases serum (1 ⟶ 3)-beta-d-glucan, without candidemia, and worsens cecal ligation and puncture sepsis in murine model. Shock. 2018;49:62–70.
Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273 (table of contents).
Helander HF, Fandriks L. Surface area of the digestive tract—revisited. Scand J Gastroenterol. 2014;49:681–689.
Sertaridou E, Papaioannou V, Kolios G, et al. Gut failure in critical care: old school versus new school. Ann Gastroenterol. 2015;28:309–322.
Carrico CJ, Meakins JL, Marshall JC, et al. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196–208.
MacFie J, O’Boyle C, Mitchell CJ, et al. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–228.
Panpetch W, Chancharoenthana W, Bootdee K, et al. Lactobacillus rhamnosus L34 attenuates gut translocation-induced bacterial sepsis in murine models of leaky gut. Infect Immun. 2018;86. https://doi.org/10.1128/IAI.00700-17.
Schmid-Schonbein GW, Chang M. The autodigestion hypothesis for shock and multi-organ failure. Ann Biomed Eng. 2014;42:405–414.
Wang GJ, Gao CF, Wei D, et al. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009;15:1427–1430.
Reintam A, Parm P, Kitus R, et al. Gastrointestinal symptoms in intensive care patients. Acta Anaesthesiol Scand. 2009;53:318–324.
Fink MP. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med. 1991;19:627–641.
Sauerwein H, van Schijndel RS. Perspective: how to evaluate studies on peri-operative nutrition? Considerations about the definition of optimal nutrition for patients and its key role in the comparison of the results of studies on nutritional intervention. Clin Nutr. 2007;26:154–158.
Doig CJ, Sutherland LR, Sandham JD, et al. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–451.
Tap J, Mondot S, Levenez F, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574–2584.
McKenney ES, Kendall MM. Microbiota and pathogen ‘pas de deux’: setting up and breaking down barriers to intestinal infection. Pathog Dis. 2016;74:ftw051. https://doi.org/10.1093/femspd/ftw051.
Cabrera-Perez J, Badovinac VP, Griffith TS. Enteric immunity, the gut microbiome, and sepsis: rethinking the germ theory of disease. Exp Biol Med (Maywood). 2017;242:127–139.
Turnbaugh PJ, Backhed F, Fulton L, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223.
Krezalek MA, DeFazio J, Zaborina O, et al. The shift of an intestinal “microbiome” to a “pathobiome” governs the course and outcome of sepsis following surgical injury. Shock. 2016;45:475–482.
Dollive S, Chen YY, Grunberg S, et al. Fungi of the murine gut: episodic variation and proliferation during antibiotic treatment. PLoS ONE. 2013;8:e71806.
Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785.
Hallen-Adams HE, Suhr MJ. Fungi in the healthy human gastrointestinal tract. Virulence. 2017;8:352–358.
Gouba N, Drancourt M. Digestive tract mycobiota: a source of infection. Med Mal Infect. 2015;45:9–16.
Lutzoni F, Kauff F, Cox CJ, et al. Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot. 2004;91:1446–1480.
Samonis G, Kofteridis DP, Maraki S, et al. Levofloxacin and moxifloxacin increase human gut colonization by Candida species. Antimicrob Agents Chemother. 2005;49:5189.
Vardakas KZ, Michalopoulos A, Kiriakidou KG, et al. Candidaemia: incidence, risk factors, characteristics and outcomes in immunocompetent critically ill patients. Clin Microbiol Infect. 2009;15:289–292.
Qiu X, Zhang F, Yang X, et al. Changes in the composition of intestinal fungi and their role in mice with dextran sulfate sodium-induced colitis. Sci Rep. 2015;5:10416.
Iliev ID, Funari VA, Taylor KD, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317.
Yamaguchi N, Sonoyama K, Kikuchi H, et al. Gastric colonization of Candida albicans differs in mice fed commercial and purified diets. J Nutr. 2005;135:109–115.
Samonis G, Maraki S, Barbounakis E, et al. Effects of vancomycin, teicoplanin, linezolid, quinupristin-dalfopristin, and telithromycin on murine gut colonization by Candida albicans. Med Mycol. 2006;44:193–196.
Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208.
Kett DH, Azoulay E, Echeverria PM, et al. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med. 2011;39:665–670.
Hedderwick SA, Lyons MJ, Liu M, et al. Epidemiology of yeast colonization in the intensive care unit. Eur J Clin Microbiol Infect Dis. 2000;19:663–670.
Kumamoto CA. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol. 2011;14:386–391.
Miranda L, Van Der Heijden I, Costa S, et al. Candida colonisation as a source for candidaemia. J Hosp Infect. 2009;72:9–16.
Kollef M, Micek S, Hampton N, et al. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54:1739–1746.
Lau AF, Kabir M, Chen SC, et al. Candida colonization as a risk marker for invasive candidiasis in mixed medical-surgical ICUs: development and evaluation of a simple, standard protocol. J Clin Microbiol. 2015;53:1324–1330.
Sam QH, Chang MW, Chai LY. The Fungal Mycobiome and Its Interaction with Gut Bacteria in the Host. Int J Mol Sci. 2017;18:E330. https://doi.org/10.3390/ijms18020330.
Panpetch W, Somboonna N, Bulan DE, et al. Oral administration of live- or heat-killed Candida albicans worsened cecal ligation and puncture sepsis in a murine model possibly due to an increased serum (1 → 3)-beta-D-glucan. PLoS ONE. 2017;12:1439.
Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2609–2618.
Juvonen PO, Alhava EM, Takala JA. Gut permeability in patients with acute pancreatitis. Scand J Gastroenterol. 2000;35:1314–1318.
Chen K, Wang Q, Pleasants RA, et al. Empiric treatment against invasive fungal diseases in febrile neutropenic patients: a systematic review and network meta-analysis. BMC Infect Dis. 2017;17:159.
Plantinga NL, de Smet A, Oostdijk EAN, et al. Selective digestive and oropharyngeal decontamination in medical and surgical ICU patients: individual patient data meta-analysis. Clin Microbiol Infect. 2018;24:505–513.
Sánchez-Ramírez C, Hípola-Escalada S, Cabrera-Santana M, et al. Long-term use of selective digestive decontamination in an ICU highly endemic for bacterial resistance. Crit Care. 2018;22:141.
Oostdijk EA, Smits L, de Smet AMG, et al. Colistin resistance in gram-negative bacteria during prophylactic topical colistin use in intensive care units. Intensive Care Med. 2013;39:653–660.
Plantinga NL, Bonten MJ. Selective decontamination and antibiotic resistance in ICUs. Crit Care. 2015;19:259.
Panpetch W, Somboonna N, Bulan DE, et al. Oral administration of live- or heat-killed Candida albicans worsened cecal ligation and puncture sepsis in a murine model possibly due to an increased serum (1 → 3)-β-D-glucan. PLoS ONE. 2017;12:e0181439.
Worasilchai N, Leelahavanichkul A, Kanjanabuch T, et al. (1 → 3)-beta-d-glucan and galactomannan testing for the diagnosis of fungal peritonitis in peritoneal dialysis patients, a pilot study. Med Mycol. 2015;53:338–346.
Leelahavanichkul A, Pongpirul K, Thongbor N, et al. (1 → 3)-beta-d-glucan and galactomannan for differentiating chemical “black particles” and fungal particles inside peritoneal dialysis tubing. Perit Dial Int. 2016;36:402–409.
Munford RS. Endotoxemia-menace, marker, or mistake? J Leukoc Biol. 2016;100:687–698.
Guerville M, Boudry G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1–G15.
Arana DM, Prieto D, Roman E, et al. The role of the cell wall in fungal pathogenesis. Microb Biotechnol. 2009;2:308–320.
Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 2009;1788:832–841.
Vojdani A. For the assessment of intestinal permeability, size matters. Altern Ther Health Med. 2013;19:12–24.
Dlugosz A, Winckler B, Lundin E, et al. No difference in small bowel microbiota between patients with irritable bowel syndrome and healthy controls. Sci Rep. 2015;5:8508.
Hofer U, Schlaepfer E, Baenziger S, et al. Inadequate clearance of translocated bacterial products in HIV-infected humanized mice. PLoS Pathog.. 2010;6:e1000867.
Ghoshal S, Witta J, Zhong J, et al. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97.
Erridge C, Attina T, Spickett CM, et al. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–1292.
Guler O, Ugras S, Aydin M, et al. The effect of lymphatic blockage on the amount of endotoxin in portal circulation, nitric oxide synthesis, and the liver in dogs with peritonitis. Surg Today. 1999;29:735–740.
van Deventer SJ, ten Cate JW, Tytgat GN. Intestinal endotoxemia. Clinical significance. Gastroenterology. 1988;94:825–831.
Dickson RP, Singer BH, Newstead MW, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1:16113.
Moore FA, Moore EE, Poggetti R, et al. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991;31:629–636 (discussion 36-8).
Yoshida M, Roth RI, Grunfeld C, et al. Soluble (1 → 3)-beta-d-glucan purified from Candida albicans: biologic effects and distribution in blood and organs in rabbits. J Lab Clin Med. 1996;128:103–114.
Rice PJ, Lockhart BE, Barker LA, et al. Pharmacokinetics of fungal (1-3)-beta-D-glucans following intravenous administration in rats. Int Immunopharmacol. 2004;4:1209–1215.
Hutter JC, Kim CS. Physiological-based pharmacokinetic modeling of endotoxin in the rat. Toxicol Ind Health. 2014;30:442–453.
Raggam RB, Fischbach LM, Prattes J, et al. Detection of (1 → 3)-beta-d-glucan in same-day urine and serum samples obtained from patients with haematological malignancies. Mycoses. 2015;58:394–398.
Reiser J, von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Investig. 2004;113:1390–1397.
Comper WD. Is the LPS-mediated proteinuria mouse model relevant to human kidney disease? Nat Med. 2009;15:133 (author reply-4).
Wei C, Moller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63.
Matsumoto T, Tanaka M, Ogata N, et al. Significance of urinary endotoxin concentration in patients with urinary tract infection. Urol Res. 1991;19:293–295.
Boelke E, Jehle PM, Storck M, et al. Urinary endotoxin excretion and urinary tract infection following kidney transplantation. Transpl Int. 2001;14:307–310.
Chung H, Pamp SJ, Hill JA, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593.
Sprinz H, Kundel DW, Dammin GJ, et al. The response of the germfree guinea pig to oral bacterial challenge with Escherichia coli and Shigella flexneri. Am J Pathol. 1961;39:681–695.
Sedman PC, Macfie J, Sagar P, et al. The prevalence of gut translocation in humans. Gastroenterology. 1994;107:643–649.
Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta Int J Clin Chem. 2002;323:59–72.
Zou B, Jiang W. Acyloxyacyl hydrolase promotes the resolution of lipopolysaccharide-induced acute lung injury. PLoS Pathog. 2017;13:e1006436.
Feulner JA, Lu M, Shelton JM, et al. Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract. Infect Immun. 2004;72:3171–3178.
Lei W, Ni H, Herington J, et al. Alkaline phosphatase protects lipopolysaccharide-induced early pregnancy defects in mice. PLoS One. 2015;10:e0123243.
Leelahavanichkul A, Panpetch W, Worasilchai N, et al. Evaluation of gastrointestinal leakage using serum (1 → 3)-beta-d-glucan in a Clostridium difficile murine model. FEMS Microbiol Lett. 2016;363:fnw204. https://doi.org/10.1093/femsle/fnw204.
Eggimann P, Pittet D. Candida colonization index and subsequent infection in critically ill surgical patients: 20 years later. Intensive Care Med. 2014;40:1429–1448.
Noss I, Doekes G, Thorne PS, et al. Comparison of the potency of a variety of beta-glucans to induce cytokine production in human whole blood. Innate Immun. 2013;19:10–19.
Ferwerda G, Meyer-Wentrup F, Kullberg BJ, et al. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–2066.
Dennehy KM, Ferwerda G, Faro-Trindade I, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506.
Bolland S, Yim YS, Tus K, et al. Genetic modifiers of systemic lupus erythematosus in FcgammaRIIB(-/-) mice. J Exp Med. 2002;195:1167–1174.
Ondee T, Surawut S, Taratummarat S, et al. Fc gamma receptor IIB deficient mice: a lupus model with increased endotoxin tolerance-related sepsis susceptibility. Shock. 2017;47:743–752.
Vogelpoel LT, Hansen IS, Rispens T, et al. Fc gamma receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages. Nat Commun. 2014;5:5444.
Kingeter LM, Lin X. C-type lectin receptor-induced NF-kappaB activation in innate immune and inflammatory responses. Cell Mol Immunol. 2012;9:105–112.
Karsten CM, Pandey MK, Figge J, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 2012;18:1401–1406.
Issara-Amphorn J, Surawut S, Worasilchai N, et al. The synergy of endotoxin and (1 → 3)-beta-D-glucan, from gut translocation, worsens sepsis severity in a lupus model of fc gamma receptor IIb-deficient mice. J Innate Immun. 2018;10:189–201.
Netea MG, Joosten LA, Latz E, et al. Trained immunity: a program of innate immune memory in health and disease. Science (New York, NY). 2016;352:aaf1098.
Bashir KM, Choi J-S. Clinical and physiological perspectives of β-glucans: the past, present, and future. Int J Mol Sci. 2017;18:1906.
Strnad P, Tacke F, Koch A, et al. Liver—guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66.
Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524.
Luther J, Garber JJ, Khalili H, et al. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell Mol Gastroenterol Hepatol. 2015;1:222–232.
Acharya C, Bajaj JS. Altered microbiome in patients with cirrhosis and complications. Clin Gastroenterol Hepatol. 2018;17:307–321.
Yang AM, Inamine T, Hochrath K, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Investig. 2017;127:2829–2841.
Bajaj JS, Thacker LR, Fagan A, et al. Gut microbial RNA and DNA analysis predicts hospitalizations in cirrhosis. JCI Insight. 2018;3:98019. https://doi.org/10.1172/jci.insight.98019.
Fukui H. Gut-liver axis in liver cirrhosis: how to manage leaky gut and endotoxemia. World J Hepatol. 2015;7:425–442.
Doi K. Role of kidney injury in sepsis. J Intensive Care. 2016;4:17.
White LE, Hassoun HT, Bihorac A, et al. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. J Trauma Acute Care Surg. 2013;75:432–438.
Hoste EA, Lameire NH, Vanholder RC, et al. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–1030.
Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47.
Alobaidi R, Basu RK, Goldstein SL, et al. Sepsis-associated acute kidney injury. Semin Nephrol. 2015;35:2–11.
Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49.
Cohen G, Horl WH. Immune dysfunction in uremia—an update. Toxins (Basel). 2012;4:962–990.
Le Bastard Q, Al-Ghalith GA, Gregoire M, et al. Systematic review: human gut dysbiosis induced by non-antibiotic prescription medications. Aliment Pharmacol Ther. 2018;47:332–345.
Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–670.
Ramezani A, Massy ZA, Meijers B, et al. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67:483–498.
Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315.
Cummings JH. Fermentation in the human large intestine: evidence and implications for health. Lancet (London, England). 1983;1:1206–1209.
de Loor H, Meijers BK, Meyer TW, et al. Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography. J Chromatogr A. 2009;1216:4684–4688.
Adesso S, Popolo A, Bianco G, et al. The uremic toxin indoxyl sulphate enhances macrophage response to LPS. PLoS ONE. 2013;8:e76778.
Wong J, Vilar E, Farrington K. Endotoxemia in end-stage kidney disease. Semin Dial. 2015;28:59–67.
Grant CJ, Harrison LE, Hoad CL, et al. Patients with chronic kidney disease have abnormal upper gastro-intestinal tract digestive function: a study of uremic enteropathy. J Gastroenterol Hepatol. 2017;32:372–377.
Noel S, Martina-Lingua MN, Bandapalle S, et al. Intestinal microbiota-kidney cross talk in acute kidney injury and chronic kidney disease. Nephron Clin Pract. 2014;127:139–143.
McDonald D, Ackermann G, Khailova L, et al. Extreme dysbiosis of the microbiome in critical illness. mSphere. 2016;1. https://doi.org/10.1128/mSphere.00199-16.
Lobo LA, Benjamim CF, Oliveira AC. The interplay between microbiota and inflammation: lessons from peritonitis and sepsis. Clin Transl Immunol. 2016;5:e90.
Vincent JL, Moreno R. Clinical review: scoring systems in the critically ill. Crit Care (London, England). 2010;14:207.
Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520.
Paphitou NI, Ostrosky-Zeichner L, Rex JH. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med Mycol. 2005;43:235–243.
León C, Ruiz-Santana S, Saavedra P, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34:730–737.
Ostrosky-Zeichner L, Sable C, Sobel J, et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis. 2007;26:271–276.
Xie G-H, Fang X-M, Fang Q, et al. Impact of invasive fungal infection on outcomes of severe sepsis: a multicenter matched cohort study in critically ill surgical patients. Crit Care. 2008;12:R5.
Pfaller MA, Messer SA, Moet GJ, et al. Candida bloodstream infections: comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008–2009). Int J Antimicrob Agents. 2011;38:65–69.
Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347:2020–2029.
Kuse E-R, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007;369:1519–1527.
Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007;356:2472–2482.
Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883–893.
Betts RF, Nucci M, Talwar D, et al. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis. 2009;48:1676–1684.
Neofytos D, Lu K, Hatfield-Seung A, et al. Epidemiology, outcomes, and risk factors of invasive fungal infections in adult patients with acute myelogenous leukemia after induction chemotherapy. Diagn Microbiol Infect Dis. 2013;75:144–149.
Marotta F, Barreto R, Kawakita S, et al. Preventive strategy for Candida gut translocation during ischemia–reperfusion injury supervening on protein–calorie malnutrition. Chin J Dig Dis. 2006;7:33–38.
Allert S, Förster TM, Svensson C-M, et al. Candida albicans-induced epithelial damage mediates translocation through intestinal barriers. mBio. 2018;9:e00915–e00918.
Eggimann P, Francioli P, Bille J, et al. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit Care Med. 1999;27:1066–1072.
Pelz RK, Hendrix CW, Swoboda SM, et al. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg. 2001;233:542.
Sandven P, Qvist H, Skovlund E, et al. Significance of Candida recovered from intraoperative specimens in patients with intra-abdominal perforations. Crit Care Med. 2002;30:541–547.
Garbino J, Lew DP, Romand J-A, et al. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: a randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med. 2002;28:1708–1717.
Jacobs S, Evans DAP, Tariq M, et al. Fluconazole improves survival in septic shock: a randomized double-blind prospective study. Crit Care Med. 2003;31:1938–1946.
Normand S, François B, Dardé M-L, et al. Oral nystatin prophylaxis of Candida spp. colonization in ventilated critically ill patients. Intensive Care Med. 2005;31:1508–1513.
Schuster MG, Edwards JE, Sobel JD, et al. Empirical fluconazole versus placebo for intensive care unit patients: a randomized trial. Ann Intern Med. 2008;149:83–90.
Giglio M, Caggiano G, Dalfino L, et al. Oral nystatin prophylaxis in surgical/trauma ICU patients: a randomised clinical trial. Crit Care. 2012;16:R57.
Ostrosky-Zeichner L, Shoham S, Vazquez J, et al. MSG-01: a randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis. 2014;58:1219–1226.
Knitsch W, Vincent J-L, Utzolino S, et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis. 2015;61:1671–1678.
Timsit J-F, Azoulay E, Schwebel C, et al. Empirical micafungin treatment and survival without invasive fungal infection in adults with ICU-acquired sepsis, Candida colonization, and multiple organ failure: the EMPIRICUS randomized clinical trial. JAMA. 2016;316:1555–1564.
Funding
Chulalongkorn University Office of International Affairs Scholarship for Short-term Research and Ratchadapisek Somphot Fund for Postdoctoral Fellowship, Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amornphimoltham, P., Yuen, P.S.T., Star, R.A. et al. Gut Leakage of Fungal-Derived Inflammatory Mediators: Part of a Gut-Liver-Kidney Axis in Bacterial Sepsis. Dig Dis Sci 64, 2416–2428 (2019). https://doi.org/10.1007/s10620-019-05581-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05581-y