Abstract
Background
The role of nucleos(t)ide analogs (NAs) therapy in intermediate and advanced hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) remains unclear.
Aims
The aim was to evaluate the effect of NAs therapy on survival of intermediate- and advanced-stage HBV-related HCC patients initially treated with chemoembolization.
Methods
A total of 1016 Barcelona Clinic Liver Cancer (BCLC) stage B/C HBV-related HCC patients initially treated with chemoembolization were included. Propensity score matching (PSM) was performed to decrease heterogeneity between the antiviral and non-antiviral groups. Kaplan–Meier and Cox regression analysis were performed to evaluate the effects of NAs therapy on overall survival (OS).
Results
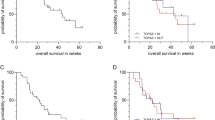
Antiviral group (n = 394) significantly prolonged OS compared with non-antiviral group (n = 622) (p = 0.003). NAs therapy (p < 0.001) along with tumor size (p = 0.002), tumor number (p = 0.001), gross vascular invasion (p < 0.001), metastasis (p < 0.001), α-fetoprotein (p < 0.001), Child–Pugh score (p = 0.008), aspartate aminotransferase (p < 0.001), and HBV DNA (p = 0.018) were identified as independent prognostic factors for OS. After PSM processing, deducting the influence of subsequent treatments for HCC, NAs therapy was still identified as an independent protective factor (p = 0.009) for OS in patients who survived ≥ 7 months, regardless of BCLC stage B or C HCC.
Conclusion
NAs therapy prolongs OS in intermediate- and advanced-stage HBV-related HCC patients initially treated with chemoembolization. After PSM processing, patients who survived ≥ 7 months still benefited from NAs therapy.





Similar content being viewed by others
References
Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology). J Gastrointest Cancer. 2017;48:238–240.
Tanaka M, Katayama F, Kato H, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol. 2011;21:401–416.
Liver EAFTSOT. EASL. Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398.
Wong JW, Wong GH, Tsoi KF, et al. Meta-analysis: the efficacy of antiviral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Therapeut. 2011;33:1104–1112.
Yang T, Lu J-H, Zhai J, et al. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol. 2012;38:683–691.
Kumada T, Toyoda H, Tada T, et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol. 2013;58:427–433.
Wu C-Y, Chen Y-J, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1913.
Zuo C, Xia M, Wu Q, Zhu H, Liu J, Liu C. Role of antiviral therapy in reducing recurrence and improving survival in hepatitis B virus-associated hepatocellular carcinoma following curative resection. Oncol Lett. 2015;9:527–534.
Yin J, Li N, Han Y, et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: a two-stage longitudinal clinical study. J Clin Oncol. 2013;31:3647–3655.
Toyoda H, Kumada T, Tada T, Sone Y, Fujimori M. Transarterial chemoembolization for hepatitis B virus-associated hepatocellular carcinoma: improved survival after concomitant treatment with nucleoside analogues. J Vasc Interv Radiol. 2012;23:317–322.
Xu X, Huang P, Tian H, et al. Role of lamivudine with transarterial chemoembolization in the survival of patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:1273–1278.
Xu L, Gao H, Huang J, et al. Antiviral therapy in the improvement of survival of patients with hepatitis B virus-related hepatocellular carcinoma treated with sorafenib. J Gastroenterol Hepatol. 2015;30:1032–1039.
Yang Y, Wen F, Li J, et al. A high baseline HBV load and antiviral therapy affect the survival of patients with advanced HBV-related HCC treated with sorafenib. Liver Int. 2015;35:2147–2154.
Zhou Z-G, Zheng X-R, Zhou Q, et al. Impact of oral anti-hepatitis B therapy on the survival of patients with hepatocellular carcinoma initially treated with chemoembolization. Chin J Cancer. 2015;34:14.
Wong GH, Tse YK, Chan HY, Yip TF, Tsoi KF, Wong VS. Oral nucleos (t) ide analogues reduce recurrence and death in chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Therapeut. 2016;43:802–813.
Liver EAFTSOT. EASL–EORTC. Clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943.
Satake M, Uchida H, Arai Y, et al. Transcatheter arterial chemoembolization (TACE) with lipiodol to treat hepatocellular carcinoma: survey results from the TACE study group of Japan. Cardiovasc Interv Radiol. 2008;31:756–761.
Bargellini I, Florio F, Golfieri R, Grosso M, Lauretti DL, Cioni R. Trends in utilization of transarterial treatments for hepatocellular carcinoma: results of a survey by the Italian Society of Interventional Radiology. Cardiovasc Interv Radiol. 2014;37:438–444.
Lao XM, Wang D, Shi M, et al. Changes in hepatitis B virus DNA levels and liver function after transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatol Res. 2011;41:553–563.
Lao XM, Luo G, Ye LT, et al. Effects of antiviral therapy on hepatitis B virus reactivation and liver function after resection or chemoembolization for hepatocellular carcinoma. Liver Int. 2013;33:595–604.
Liver EAFTSOT. EASL. Clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242.
Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38.
Williams BA. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes. 2006.
Li X, Zhong X, Chen Z-H, et al. Hepatitis B virus DNA negativity acts as a favorable prognostic factor in hepatocellular carcinoma patients. Asian Pac J Cancer Prev. 2014;15:9635–9641.
Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261–2273.
Pinter M, Sieghart W, Hucke F, et al. Prognostic factors in patients with advanced hepatocellular carcinoma treated with sorafenib. Aliment Pharmacol Therapeut. 2011;34:949–959.
Dan J-Q, Zhang Y-J, Huang J-T, et al. Hepatitis B virus reactivation after radiofrequency ablation or hepatic resection for HBV-related small hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2013;39:865–872.
Huang G, Lai EC, Lau WY, et al. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg. 2013;257:490–505.
Jang JW. Hepatitis B virus reactivation in patients with hepatocellular carcinoma undergoing anti-cancer therapy. World J Gastroenterol. 2014;20:7675.
Chen J, Lai L, Lin Q, et al. Hepatic resection after transarterial chemoembolization increases overall survival in large/multifocal hepatocellular carcinoma: a retrospective cohort study. Oncotarget. 2017;8:408.
Long J, Zheng J-S, Sun B, Lu N. Microwave ablation of hepatocellular carcinoma with portal vein tumor thrombosis after transarterial chemoembolization: a prospective study. Hepatol Int. 2016;10:175–184.
Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893.
Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475.
Funding
This work was partly supported by project grants from the National Natural Science Foundation of China (No. 81773057).
Author information
Authors and Affiliations
Contributions
XML is the correspondent author of this article and also acting as the submission’s guarantor. ZWJ, XWW, and ZXC have equally contributed to this article as first authors. XML conceived and designed the study. ZWJ, XWW, ZXC, JCW, and JYP collected and analyzed the data. ZWJ, XWW, ZXC, and XML interpreted the data. ZWJ, XWW, ZXC, and XML drafted or wrote the manuscript. XML obtained funding. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Ethics approval
Approved by the Institutional Review Board of Sun Yat-sen University Cancer Center.
Patient consent
Obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jian, ZW., Wu, XW., Chen, ZX. et al. Effect of Nucleos(t)ide Analogs on Patients with Intermediate and Advanced Hepatitis B Virus-Related Hepatocellular Carcinoma. Dig Dis Sci 64, 2187–2198 (2019). https://doi.org/10.1007/s10620-019-05543-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05543-4