Abstract
Background
The eradication of Helicobacter pylori infection remains a challenge, especially in the patients unsuitable to take penicillin. Cephalosporin has the potential to replace amoxicillin for H. pylori eradication.
Aims
To compare the effectiveness, safety, and compliance of amoxicillin- and cefuroxime-containing quadruple regimens in treatment-naïve patients.
Methods
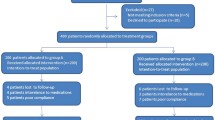
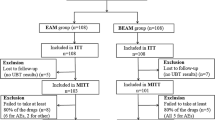
In this open-label randomized control study, 400 patients with H. pylori infection were divided into amoxicillin-containing (esomeprazole 20 mg twice/day, amoxicillin 1000 mg twice/day, levofloxacin 500 mg once/day, and bismuth 220 mg twice/day for 14 days) or cefuroxime-containing (esomeprazole 20 mg twice/day, cefuroxime 500 mg twice/day, levofloxacin 500 mg once/day, and bismuth 220 mg twice/day for 14 days) quadruple therapy groups. The safety and compliance were assessed 1–3 days after eradication. Urea breath test was performed 8–12 weeks after eradication to determine treatment outcome.
Results
The baseline data including antibiotic resistance were well matched between the two groups. The eradication rates between amoxicillin- and cefuroxime-containing quadruple therapy groups were not significantly different [intention-to-treat analysis: 83.5% (95% confidence interval 78.3–88.7%) vs. 81.0% (75.5–86.5%), P = 0.513; modified intention-to-treat analysis: 90.3% (86.0–94.6%) vs. 88.5% (83.9–93.2%), P = 0.586; per-protocol analysis: 91.6% (87.5–95.7%) vs. 89.8% (85.3–94.3%), P = 0.560]. The incidence of adverse effects (18.4 vs. 20.1%, P = 0.678) and compliance (94.7 vs. 94.2%, P = 0.813) were also similar. Variate analyses showed that antibiotic resistance and poor compliance were the independent risk factors for eradication failure.
Conclusions
Esomeprazole, bismuth, levofloxacin, and amoxicillin or cefuroxime achieved similar and relatively satisfactory cure rates, safety, and compliance in first-line H. pylori eradication. Cefuroxime may be a good alternative medicine for eradication instead of amoxicillin for the patients unsuitable to take penicillin.



Similar content being viewed by others
References
Su P, Li Y, Li H, et al. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013;18:274–279.
Song Z, Zhang J, He L, et al. Prospective multi-region study on primary antibiotic resistance of Helicobacter pylori strains isolated from Chinese patients. Dig Liver Dis. 2014;46:1077–1081.
Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42.
Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection–the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664.
Malfertheiner PMF, O’Morain CAGJP, Kuipers EJ. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30.
Liu WZ, Xie Y, Cheng H, et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14:211–221.
Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153.
Pichichero ME, Zagursky R. Penicillin and cephalosporin allergy. Ann Allergy Asthma Immunol. 2014;112:404–412.
Mirakian R, Leech SC, Krishna MT, et al. Management of allergy to penicillins and other beta-lactams. Clin Exp Allergy. 2015;45:300–327.
Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin-allergic patients: A meta-analysis. Otolaryngol Head Neck Surg. 2007;136:340–347.
Tatsuta M, Ishikawa H, Iishi H, Okuda S, Yokota Y. Reduction of gastric ulcer recurrence after suppression of Helicobacter pylori by cefixime. Gut. 1990;31:973–976.
Fagoonee S, Astegiano M, Smedile A, Pellicano R. Efficacy of cefixime-based triple therapy for Helicobacter pylori eradication: A retrospective study. Panminerva Med. 2013;55:309–310.
Bai P, Zhou LY, Xiao XM, Luo Y, Ding Y. Susceptibility of Helicobacter pylori to antibiotics in Chinese patients. J Dig Dis. 2015;16:464–470.
Campagna JD, Bond MC, Schabelman E, Hayes BD. The use of cephalosporins in penicillin-allergic patients: A literature review. J Emerg Med. 2012;42:612–620.
Pichichero ME. Use of selected cephalosporins in penicillin-allergic patients: A paradigm shift. Diagn Microbiol Infect Dis. 2007;57:13S–18S.
Miranda A, Blanca M, Vega JM, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol. 1996;98:671–677.
Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181.
Zhou L, Zhang J, Song Z, et al. Tailored versus triple plus bismuth or concomitant therapy as initial Helicobacter pylori treatment: A randomized trial. Helicobacter. 2016;21:91–99.
Zhou L, Zhang J, Chen M, et al. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: A randomized multicenter trial. Am J Gastroenterol. 2014;109:535–541.
Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut. 2015;64:1715–1720.
Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145:121.e1–128.e1.
Liou JM, Chen CC, Chen MJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: A multicentre, open-label, randomised trial. Lancet. 2013;381:205–213.
Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: Lessons from China. Eur J Gastroenterol Hepatol. 2013;25:1134–1140.
Liao J, Zheng Q, Liang X, et al. Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter. 2013;18:373–377.
Mégraud F, Bénéjat L, Ontsira NEN, Lehours P. Molecular approaches to identify Helicobacter pylori antimicrobial resistance. Gastroenterol Clin N Am. 2015;44:577–596.
Samra Z, Shmuely H, Niv Y, et al. Resistance of Helicobacter pylori isolated in Israel to metronidazole, clarithromycin, tetracycline, amoxicillin and cefixime. J Antimicrob Chemother. 2002;49:1023–1026.
Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: Effect of resistance, duration, and CYP2C19 genotype. Helicobacter. 2016;21:85–90.
Marcus EA, Sachs G, Scott DR. Colloidal bismuth subcitrate impedes proton entry into Helicobacter pylori and increases the efficacy of growth-dependent antibiotics. Aliment Pharmacol Ther. 2015;42:922–933.
Gisbert JP, Perez-Aisa A, Bermejo F, et al. Second-line therapy with levofloxacin after failure of treatment to eradicate Helicobacter pylori infection: Time trends in a Spanish Multicenter Study of 1000 patients. J Clin Gastroenterol. 2013;47:130–135.
Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65:870–878.
Acknowledgments
The study was supported by the National Science & Technology Pillar Program of twelfth Five-Year Plan in China (2012BAI06B02) and the key laboratory for Helicobacter pylori infection and upper gastrointestinal diseases in Beijing (No. BZ0371). The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author information
Authors and Affiliations
Contributions
WF contributed to the clinical studies and experimental studies. ZS contributed to the study concept, study design, clinical studies, and manuscript writing. LZ contributed to the study concept, study design, clinical studies, and manuscript editing. YX contributed to the clinical studies. YD contributed to the experimental studies. BS contributed to the clinical studies. XT contributed to the clinical studies. LW contributed to the clinical studies. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest in the submission of this manuscript.
Rights and permissions
About this article
Cite this article
Fu, W., Song, Z., Zhou, L. et al. Randomized Clinical Trial: Esomeprazole, Bismuth, Levofloxacin, and Amoxicillin or Cefuroxime as First-Line Eradication Regimens for Helicobacter pylori Infection. Dig Dis Sci 62, 1580–1589 (2017). https://doi.org/10.1007/s10620-017-4564-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4564-4