Abstract
Background
Vedolizumab (VDZ) has demonstrated long-term efficacy in Crohn’s disease (CD) and ulcerative colitis (UC) in phase III trials.
Aims
Our aim was to evaluate the efficacy of VDZ at week 54 in inflammatory bowel disease (IBD) in a multicenter cohort of patients.
Methods
Adult patients completing induction therapy with VDZ were eligible for this study. Clinical response and remission was assessed using the Harvey–Bradshaw Index (HBI) for CD, the Simple Clinical Colitis Activity Index for UC and physician assessment.
Results
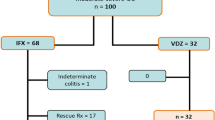
Among 136 total patients (96 CD and 40 UC), 76 (56%) demonstrated clinical response or remission at week 54. In univariate analysis, for patients with CD concomitant initiation of immunomodulator therapy (2.71, 95% CI 1.11–6.57), the addition of an immunomodulator (OR 11.49, 3.16–41.75) and CRP < 3 (4.92, 95% CI 1.99–12.15) was associated with increased odds of clinical response or remission at week 54. For UC patients, hospitalization after VDZ induction was associated with decreased odds of response or remission at week 54 (OR 0.22, 95% CI 0.05–0.88). On multivariate analysis in CD, addition of an immunomodulator (OR 8.33, 95% CI 2.15–32.26) remained significant predictors of clinical response or remission at week 54.
Conclusions
Among a multicenter cohort of patients with IBD demonstrating primary response to VDZ, the addition of combination therapy with an immunomodulator is a significant predictor of clinical response or remission at week 54 in patients with CD.
Similar content being viewed by others
References
Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohn’s Colitis. 2010;4:355–366.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Sandborn WJ, Van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257.e3–265.e3.
Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab. Ann Intern Med. 2007;146:829.
Soler D, Chapman T, Yang L-L, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875.
Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721.
Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710.
Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2016. doi:10.1136/gutjnl-2015-311079.
Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;315:514.
Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32.
Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis. 2015;21:2879–2885.
Colombel J-F, Loftus EV, Siegel CA, et al. P528. Efficacy of vedolizumab with concomitant corticosteroid or immunomodulator use in patients with Crohn’s disease in GEMINI 2. J Crohn’s Colitis. 2015;9:S344.
Colombel J-F, Loftus EV, Siegel CA, et al. P433. Efficacy of vedolizumab with concomitant corticosteroid or immunomodulator use in patients with ulcerative colitis from GEMINI 1. J Crohn’s Colitis. 2015;9:S296–S297.
Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics–pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42:188–202.
Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate–severe Crohn’s disease: results from the US VICTORY consortium. Am J Gastroenterol. 2016;111:1147–1155.
Yarur AJ, Kubiliun MJ, Czul F, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13:1118.e3–1124.e3.
Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment had failed. Gastroenterology. 2014;147:618.e3–627.e3.
Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002–1007. (quiz e78).
Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology. 2015;148:344–354.
McLean LP, Cross RK. Integrin antagonists as potential therapeutic options for the treatment of Crohn’s disease. Expert Opin Investig Drugs. 2016;25:263–273.
Dulai PS, Mosli M, Khanna R, Levesque BG, Sandborn WJ, Feagan BG. Vedolizumab for the treatment of moderately to severely active ulcerative colitis. Pharmacotherapy. 2015;35:412–423.
Hanauer SB. Positioning biologic agents in the treatment of Crohn’s disease. Inflamm Bowel Dis. 2009;15:1570–1582.
Author’s contribution
JA and EB were involved in study concept and design, analysis and interpretation of data, critical revision of the manuscript. MS and BS involved in acquisition of data. VY, AA and JK involved in study concept and design, revision of manuscript. All authors approved the final version of this manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ananthakrishnan has served on scientific advisory boards for Abbvie and Cubist. Yajnik has been a consultant for Janssen, UCB, Takeda and Abbvie. Korzenik is supported by funding from Takeda. None of the other authors have conflicts of interest to declare.
Guarantor
Jessica R. Allegretti.
Additional information
Jessica R. Allegretti and Edward L. Barnes have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Allegretti, J.R., Barnes, E.L., Stevens, B. et al. Predictors of Clinical Response and Remission at 1 Year Among a Multicenter Cohort of Patients with Inflammatory Bowel Disease Treated with Vedolizumab. Dig Dis Sci 62, 1590–1596 (2017). https://doi.org/10.1007/s10620-017-4549-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4549-3