Abstract
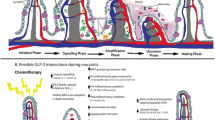
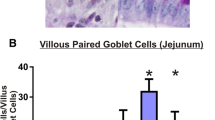
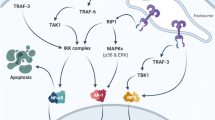
The alimentary tract mucosal inflammation has been a topic of concern in oncology; though many modalities of treatment have been proposed for mucosal inflammation, the contributing adverse effects have severely affected the quality of life of patients. This review focuses on the importance of neurogenic peptide, Substance P and its receptor NK-1R in modulating the cascades of events in mucosal inflammation during cytotoxic therapy. There are various preclinical and clinical models showing increased expression of Substance P/NK-1R in ionizing radiation and chemotherapy, but only very few preclinical studies to our knowledge have highlighted or examined its role in mucosal inflammation. Hence, the importance of neuropeptide involved in the inflammatory events in mucosal inflammation in cytotoxic therapy could be a major breakthrough for future research purposes and treatment. The factors contributing to the severity of tissue reactions have been multietiogenic; thus, resultant treatment also has to be directed toward multiple contributing factors. This review also focuses on the significance of care strategy to be adopted in alimentary tract mucositis when multietiogenic factors are taken into consideration.


Similar content being viewed by others
References
Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68:1110–1120.
Kim JW, Cha Y, Kim SJ, et al. Association of oral mucositis with quality of life and symptom clusters in patients with solid tumors receiving chemotherapy. Support Care Cancer. 2012;20:395–403.
Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Cancer. 2008;113:2704–2713.
Sonis ST. Oral mucositis in head and neck cancer. Am Soc Clin Oncol Educ Book. 2013;2013:236–240.
Sonis ST. A biological approach to mucositis. J Support Oncol. 2004;2:21–32.
Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163–171.
Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2010;102:1188–1198.
Al-Dasooqi N, Sonis ST, Bowen JM, et al. Emerging evidence on the pathobiology of mucositis. Support Care Cancer. 2013;21:2075–2083.
Matsumoto K, Nakajima T, Sakai H, et al. Increased expression of 5-HT3 and NK 1 receptors in 5-fluorouracil-induced mucositis in mouse jejunum. Dig Dis Sci. 2013;58:3440–3451.
Höckerfelt U, Franzén L, Kjörell U, Forsgren S. Parallel increase in substance P and VIP in rat duodenum in response to irradiation. Peptides. 2000;21:271–281.
Höckerfelt U, Franzén L, Forsgren S. Substance P (NK1) receptor in relation to substance P innervation in rat duodenum after irradiation. Regul Pept. 2001;98:115–126.
Forsgren S, Höckerfelt U, Norrgård O, Henriksson R, Franzén L. Pronounced substance P innervation in irradiation-induced enteropathy—a study on human colon. Regul Pept. 2000;88:1–13.
Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant protocol 052 study group. J Clin Oncol. 2003;21:4112–4119.
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97:3090–3098.
Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23:2822–2830.
Koon HW, Pothoulakis C. Immunomodulatory properties of substance P: the gastrointestinal system as a model. Ann N Y Acad Sci. 2006;1088:23–40.
Gerard NP, Garraway LA, Eddy RL Jr, et al. Human substance P receptor (NK-1): organization of the gene, chromosome localization, and functional expression of cDNA clones. Biochemistry. 1991;30:10640–10646.
Harrison S, Geppetti P. Substance p. Int J Biochem Cell Biol. 2001;33:555–576.
Datar P, Srivastava S, Coutinho E, Govil G. Substance P: structure, function, and therapeutics. Curr Top Med Chem. 2004;4:75–103.
O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–180.
González Moles MA, Esteban F, Ruiz-Avila I, et al. A role for the substance P/NK-1 receptor complex in cell proliferation and apoptosis in oral lichen planus. Oral Dis. 2009;15:162–169.
Evangelista S. Involvement of tachykinins in intestinal inflammation. Curr Pharm Des. 2001;7:19–30.
Carter MS, Krause JE. Structure, expression, and some regulatory mechanisms of the rat preprotachykinin gene encoding substance P, neurokinin A, neuropeptide K, and neuropeptide gamma. J Neurosci. 1990;10:2203–2214.
Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat Immunol. 2000;1:392–397.
Dornan WA, Vink KL, Malen P, Short K, Struthers W, Barrett C. Site-specific effects of intracerebral injections of three neurokinins (neurokinin A, neurokinin K, and neurokinin gamma) on the expression of male rat sexual behavior. Physiol Behav. 1993;54:249–258.
Duffy RA, Hedrick JA, Randolph G, et al. Centrally administered Hemokinin-1 (HK-1), a neurokinin NK1 receptor agonist, produces substance P-like behavioral effects in mice and gerbils. Neuropharmacology. 2003;45:242–250.
Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34:39–43.
Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025.
Sonis ST. The biologic role for nuclear factor-kappa B in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit Rev Oral Biol Med. 2002;13:380–389.
Logan RM, Gibson RJ, Sonis ST, Keefe DMK. Nuclear factor-κB (NF-κB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007;43:395–401.
Sonis ST. Oral mucositis. Anticancer Drugs. 2011;22:607–612.
Kozakiewicz M, Godlewski A. Modulation of the mitotic activity and population of the mast cells in the oral mucosa by substance P. Cell Mol Biol Lett. 2003;8:727–734.
Aalto Y, Forsgren S, Kjörell U, Franze´n L, Gustafsson H, Henriksson R. Time- and dose-related changes in the expression of substance P in salivary glands in response to fractionated irradiation. Int J Radiat Oncol Biol Phys. 1995;33:297–305.
Forsgren S, Franzén L, Funegård U, Gustafsson H, Henriksson R. Bilateral irradiation of head and neck induces an enhanced expression of substance P in the parasympathetic innervation of the submandibular gland. Neuroscience 1992;46:233–240.
Lidegran M, Domeij S, Dalqvist Å, et al. Irradiation influences the expression of substance P and enkephalin in the rat larynx. Cell Tissue Res. 1995;279:55–63.
Alfieri AB, Cubeddu LX. Efectos de los antagonistas de los receptors NK1 y de la dexametasona sobre la inflamación neurogénica inducida porciclofosfamida y por radiación X, en la rata. AVFT. 2004;23:61–66.
Goode T, O’Connell J, Sternini C, et al. Substance P (neurokinin-1) receptor is a marker of human mucosal but not peripheral mononuclear cells: molecular quantitation and localization. J Immunol. 1998;161:2232–2240.
Pothoulakis C, Castagliuolo I, Leeman SE, et al. Substance P receptor expression in intestinal epithelium in clostridium difficile toxin A enteritis in rats. Am J Physiol. 1998;275:G68–G75.
Andrews PLR, Rudd JA. The role of tachykinins and the tachykinin NK1 receptor in nausea and emesis. In: Hofmann FB, ed. Handbook of experimental pharmacology. Berlin: Springer; 2004:359–440.
González Moles MA, Mosqueda-Taylor A, Esteban F et al. Cell proliferation associated with actions of the substance P/NK-1 receptor complex in keratocystic odontogenic tumours. Oral Oncol. 2008; 44:1127–1133.
Kabashima H, Nagata K, Maeda K, Iijima T. Involvement of substance P, mast cells, TNF-alpha and ICAM-1 in the infiltration of inflammatory cells in human periapical granulomas. J Oral Pathol Med. 2002;31:175–180.
Cocchiara R, Albeggiani G, Azzolina A, et al. Effect of Substance P on uterine mast cell cytokine release during the reproductive cycle. J Neuroimmunol. 1995;60:107–115.
Cocchiara R, Bongiovanni A, Albeggiani G, et al. Inhibitory effect of neuraminidase on SP-induced histamine release and TNF-α mRNA: evidence of a receptor-independent mechanism. J Neuroimmunol. 1997;75:9–18.
Kaltschmidt B, Baeuerle PA, Kaltschmidt C. Potential involvement of the transcription factor NF-kappa B in neurological disorders. Mol Aspects Med. 1993;14:171–190.
Azzolina A, Bongiovanni A, Lampiasi N. Substance P induces TNF-alpha and IL-6 production through NF kappa B in peritoneal mast cells. Biochim Biophys Acta Mol Cell Res. 2003;1643:75–83.
Cocchiara R, Bongiovanni A, Albeggiani G, Azzolina A, Geraci D. Substance P selectively activates TNF-alpha mRNA in rat uterine immune cells: a neuroimmune link. NeuroReport. 1997;8:2961–2964.
Azzolina A, Guarneri P, Lampiasi N. Involvement of p38 and JNK MAPKs pathways in substance P-induced production of the TNF-α by peritoneal mast cells. Cytokine. 2002;18:72–80.
Ishizuka T, Terada N, Gerwins P, et al. Mast cells tumor necrosis factor alpha production is regulated by MEK kinases. Proc Natl Acad Sci. 1997;94:6358–6363.
Lorenz D, Wiesner B, Zipper J, et al. Mechanism of peptide-induced mast cell degranulation. Translocation and patch-clamp studies. J Gen Physiol. 1998;112:577–591.
Ferry X, Eichwald V, Daeffler L, Landry Y. Activation of betagamma subunits of Gi2 and Gi3 proteins by basic secretagogues induces exocytosis through phospholipases cβ and arachidonate release through phospholipase Cgamma in mast cells. J Immunol. 2001;167:4805–4813.
Page S, Fischer C, Baumgartner B, et al. 4-Hydroxynonenal prevents NFκB activation and tumor necrosis factor expression by inhibiting IκB phosphorylation and subsequent proteolysis. J Biol Chem. 1999;274:11611–11618.
Pelletier C, Varin-Blank N, Rivera J, et al. FcεRI-mediated induction of TNF-α gene expression in the RBL-2H3 mast cell line: regulation by a novel NFκB-like nuclear binding complex. J Immunol. 1998;161:4768–4776.
Al-Dasooqi N, Gibson RJ, Bowen JM, Logan RM, Stringer AM, Keefe DM. Matrix metalloproteinases are possible mediators for the development of alimentary tract mucositis in the dark agouti rat. Exp Biol Med (Maywood). 2010;235:1244–1256.
Cury PR, Canavez F, de Araújo VC, Furuse C, de Araújo NS. Substance P regulates the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in cultured human gingival fibroblasts. J Periodontal Res. 2008;43:255–260.
Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J Biol Chem. 1999;274:30858–30863.
Zampetaki A, Mitsialis SA, Pfeilschifter J, Kourembanas S. Hypoxia induces macrophage inflammatory protein-2 (MIP-2) gene expression in murine macrophages via NF-kappaB: the prominent role of p42/p44 and PI3 kinase pathways. FASEB J. 2004;18:1090–1092.
Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40.
Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441.
Kefaloyianni E, Gaitanaki C, Beis I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell Signal. 2006;18:2238–2251.
Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869.
Alarcon-Vargas D, Ronai Z. c-Jun-NH2 kinase (JNK) contributes to the regulation of c-Myc protein stability. J Biol Chem. 2004;279:5008–5016.
Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713–725.
Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112.
Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737.
Chen Z, Gibson TB, Robinson F, et al. MAP kinases. Chem Rev. 2001;101:2449–2476.
Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. 2008;294:C1586–C1596.
Maes M, Lin AH, Delmeire L, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45:833–839.
Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain Behav Immun. 2013;30:S41–S47.
Peters EM, Liotiri S, Bodo E, et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171:1872–1886.
Chancellor-Freeland C, Zhu GF, Kage R, Beller DI, Leeman SE, Black PH. Substance P and stress-induced changes in macrophages. Ann N Y Acad Sci. 1995;771:472–484.
Paus R, Heinzelmann T, Schultz KD, Furkert J, Fechner K, Czar-netzki BM. Hair growth induction by substance P. Lab Invest. 1994;71:134–140.
Peters EM, Botchkarev VA, Botchkareva NV, Tobin DJ, Paus R. Hair-cycle-associated remodelling of the peptidergic innervation of murine skin, and hair growth modulation by neuropeptides. J Invest Dermatol. 2001;116:236–245.
Peters EM, Kuhlmei A, Tobin DJ, Muller-Rover S, Klapp BF, Arck PC. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–262.
Rice FL, Fundin B T, Arvidsson J, Aldskogius H, Johansson O. Comprehensive immunofluorescence and lectin binding analysis of vibrissal follicle sinus complex innervation in the mystacial pad of the rat. J Comp Neurol. 1997: 385:149–184.
Arck PC, Handjiski B, Peters EM, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162:803–814.
Raithel M, Schneider HT, Hahn EG. Effect of substance P on histamine secretion from gut mucosa in inflammatory bowel disease. Scand J Gastroenterol. 1999;34:496–503.
Mantyh PW, Catton M, Maggio JE, Vigna SR. Alterations in receptors for sensory neuropeptides in human inflammatory bowel disease. Adv Exp Med Biol. 1991;298:253–283.
Mazumdar S, Das KM. Immunocytochemical localization of vasoactive intestinal peptide and substance P in the colon from normal subjects and patients with inflammatory bowel disease. Am J Gastroenterol. 1992;87:176–181.
Newson B, Dahlstrom A, Enerbäck L, Ahlman H. Suggestive evidence for a direct innervation of mucosal mast cells. Neuroscience. 1983;10:565–570.
Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115.
Farhadi A, Fields JZ, Keshavarzian A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots: stress, intestinal hyperpermeability and inflammation. World J Gastroenterol. 2007;13:3027–3030.
Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702.
He SH. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol. 2004;10:309–318.
Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272.
Schemann M. Control of gastrointestinal motility by the “gut brain”—the enteric nervous system. J Pediatr Gastroenterol Nutr. 2005;41:S4–S6.
Ader R, Cohen N, Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet. 1995;345:99–103.
Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869.
Reichlin S. Neuroendocrine-immune interactions. N Engl J Med. 1993;329:1246–1253.
Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491.
Barnes PJ. Molecular mechanisms of corticosteroids in allergic diseases. Allergy. 2001;56:928–936.
Ray A, Sehgal PB. Cytokines and their receptors: molecular mechanism of interleukin-6 gene repression by glucocorticoids. J Am Soc Nephrol. 1992;2:S214–S221.
Joyce DA, Gimblett G, Steer JH. Targets of glucocorticoid action on TNF- alpha release by macrophages. Inflamm Res. 2001;50:337–340.
Franchimont D, Kino T, Galon J, Meduri GU, Chorousos G. Glucocorticoids and inflammation revisited: the state of the art. NIH Clinical Staff Conference. Neuroimmunomodulation 2002–2003;10:247–260.
Yamaguchi M, Takizawa T, Nakajima R, Imamura R, Kasai. The Damon System and release of substance P in gingival crevicular fluid during orthodontic tooth movement in adults. World J Orthod.. 2009;10:141–146.
Caviedes-Bucheli J, Azuero-Holguin MM, Gutierrez-Sanchez L, et al. The effect of three different rotary instrumentation systems on substance P and calcitonin gene-related peptide expression in human periodontal ligament. J Endod. 2010;36:1938–1942.
Herpfer I, Lieb K. Substance P receptor antagonists in psychiatry: rationale for development and therapeutic potential. CNS Drugs. 2005;19:275–293.
Pike JL, Smith TL, Hauger RL, et al. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom Med. 1997;59:447–457.
Liu L, Markus I, Saghire HE, Perera DS, King DW, Burcher E. Gene expression in ulcerative colitis, Crohn’s disease and diverticular disease: a role for hemokinin-1? Neurogastroenterol Motil. 2011;23:475–483 (e179–1e80).
Morse HR, Olomolaiye OO, Wood NA, Keen LJ, Bidwell JL. Induced heteroduplex genotyping of TNF-alpha, IL-1beta, IL-6 and IL-10 polymorphisms associated with transcriptional regulation. Cytokine. 1999;11:789–795.
Andreassen CN, Alsner J, Overgaard J. Does variability in normal tissue reactions after radiotherapy have a genetic basis—where and how to look for it? Radiother Oncol. 2002;64:131–140.
Stringer AM. Interaction between host cells and microbes in chemotherapy-induced mucositis. Nutrients.. 2013;5:1488–1499.
Stringer AM, Gibson RJ, Logan RM, et al. Gastrointestinal microflora and mucins may play a critical role in the development of 5-Fluorouracil-induced gastrointestinal mucositis. Exp Biol Med (Maywood).. 2009;234:430–441.
Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284.
Graham GJ, Stevens JM, Page NM, et al. Tachykinins regulate the function of platelets. Blood. 2004;104:1058–1065.
Esteban F, Munoz M, Gonzalez-Moles MA, Rosso M. A role for substance P in cancer promotion and progression: a mechanism to counteract intracellular death signals following oncogene activation or DNA damage. Cancer Metastasis Rev. 2006;25:137–145.
Esteban F, Gonzalez-Moles MA, Castro D, et al. Expression of substance P and neurokinin-1-receptor in laryngeal cancer: linking chronic inflammation to cancer promotion and progression. Histopathology. 2009;54:258–260.
Acknowledgments
No grant support and no financial arrangements related to the research or assistance with manuscript preparation.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satheeshkumar, P.S., Mohan, M.P. Tachykinin Peptide, Substance P, and Its Receptor NK-1R Play an Important Role in Alimentary Tract Mucosal Inflammation During Cytotoxic Therapy. Dig Dis Sci 59, 2864–2873 (2014). https://doi.org/10.1007/s10620-014-3263-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3263-7