Abstract
Background
Evidence shows a strong relationship between KRAS mutations and the NF-κB signaling pathway. In colorectal cancer, however, the study of this subject has been very limited and results are inconsistent.
Aims
To examine the relationship between KRAS mutations and NF-κB activation and their effect on chemotherapy response and survival of colorectal cancer patients.
Materials and Methods
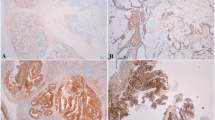
NF-κB activation was analyzed by immunohistochemistry in 167 primary colorectal cancer specimens in which the KRAS mutation status was confirmed. Clinical and pathologic data were extracted from the medical records and reviewed.
Results
Of 167 tumors screened, 63 (37.7 %) had NF-κB activation, 59 (35.3 %) had KRAS mutations, and 30 (18.0 %) had both NF-κB activation and KRAS mutations. The frequency of NF-κB activation in tumors with KRAS mutations was significantly higher than in tumors with wild type KRAS; 50.8 versus 30.6 %, P = 0.012. Patients with both KRAS mutations and NF-κB activation had a lower objective response to first-line chemotherapy than patients with other tumors, 23.8 versus 49.4 % (P = 0.035). Compared to patients with both KRAS mutations and NF-κB activation, overall survival of patients in other groups was significantly higher; median overall survival was 28.4 months (95 % CI 21.0–35.8) versus 46.3 months (95 % CI 39.4–53.2), hazard ratio 0.259 (95 % CI 0.125–0.538), P = 0.005.
Conclusions
NF-κB activation was associated with KRAS mutation, and both KRAS mutation and NF-κB activation were indicative of high tolerance of chemotherapy and poor prognosis for colorectal cancer patients. Tumors with KRAS mutations and NF-κB activation may be a unique subtype of colorectal cancer.


Similar content being viewed by others
References
Bos JL, Fearon ER, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297.
Artale S, Sartore-Bianchi A, Veronese SM, et al. Mutations in KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J Clin Oncol. 2008;26:4217–4219.
Lievre A, Bachet J-B, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treatment with cetuximab. J Clin Oncol. 2008;26:374–379.
Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362.
Maeda S, Omata M. Inflammation and cancer: role of NF-kappaB activation. Cancer Sci. 2008;99:836–842.
Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674.
Sakamoto K, Maeda S. Targeting NF-kappaB for colorectal cancer. Expert Opin Ther Targets. 2010;14:593–601.
Rakitina TV, Vasilevskaya IA, Dwyer PJ. Additive interaction of oxaliplatin and 17-allylamino-17-demethoxygeldanamycin in colon cancer cell lines results from inhibition of NF κB signaling. Cancer Res. 2003;63:8600–8605.
Camp RR, Li J, Minnich DJ, et al. Inducible NF kB activation contributes to chemotherapy resistance in gastric cancer. J Am Coll Surg. 2004;199:249–258.
Uetsuka H, Haisa M, Kimura M, et al. Inhibition of inducible NF-kappaB activity reduces chemoresistance to 5-fluorouracil in human stomach cancer cell line. Exp Cell Res. 2003;289:27–35.
Abdel-Latif MM, O’Riordan J, Windle HJ, et al. NF-kappaB activation in esophageal adenocarcinoma relationship to Barret’s metaplasia, survival and response to neoadjuvant chemoradiotherapy. Ann Surg. 2004;239:491–500.
Kojima M, Morisaki T, Sasaki N, et al. Increased NF-kB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004;24:675–681.
Izzo JG, Malhotra U, Wu T–T, et al. Association of activated factor NF κB with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol. 2006;24:748–754.
Scartozzi M, Bearzi I, Pierantoni C, et al. NF-κB tumor expression predicts response and survival in irinotecan-refractory metastatic colorectal cancer treated with cetuximab-irinotecan therapy. J Clin Oncol. 2007;25:3930–3935.
Wong KK, Jacks T, Dranoff G. NF-kappaB fans the flames of lung carcinogenesis. Cancer Prev Res (Phila). 2010;3:403–405.
Finco TS, Westwick JK, Norris JL, et al. Oncogenic Ha-Ras-induced signaling activates NF-kappaB transcriptional activity, which is required for cellular transformation. J Biol Chem. 1997;272:24113–24116.
Arsura M, Mercurio F, Oliver AL, et al. Role of the IkappaB kinase complex in oncogenic Ras- and Raf-mediated transformation of rat liver epithelial cells. Mol Cell Biol. 2000;20:5381–5391.
Mayo MW, Wang CY, Cogswell PC, et al. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815.
Jo H, Zhang R, Zhang H, et al. NF-kappa B is required for H-ras oncogene induced abnormal cell proliferation and tumorigenesis. Oncogene. 2000;19:841–849.
Bassres DS, Ebbs A, Levantini E, et al. Requirement of the NF-kappaB subunit p65/RelA for KRAS-induced lung tumorigenesis. Cancer Res. 2010;70:3537–3546.
Shen Y, Ye Y, Zheng X, Li C, Chen Q. KRAS mutations in the plasma of colorectal cancer patients. Lab Med. 2010;41:156–158.
Lind DS, Hochwald SN, Malaty J, et al. NF-kappa B is upregulated in colorectal cancer. Surgery. 2001;130:363–369.
Yu HG, Yu LL, Yang Y, et al. Increased expression of RelA/NF-kappa B protein correlates with colorectal tumorigenesis. Oncology. 2003;65:37–45.
Barbie DA, Tamayo P, Boehm JS, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112.
Cadoret A, Bertrand F, Baron-Delage S, et al. Down-regulation of NF-kappaB activity and NF-kappaB p65 subunit expression by ras and polyoma middle T oncogenes in human colonic Caco-2 cells. Oncogene. 1997;14:1589–1600.
Evertsson S, Sun XF. Protein expression of NF-kappaB in human colorectal adenocarcinoma. Int J Mol Med. 2002;10:547–550.
Deschoolmeester V, Baay M, Specenier P, Lardon F, Vermorken JB. A review of the most promising biomarkers in colorectal cancer: one step closer to targeted therapy. Oncologist. 2010;15:699–731.
Brandi G, Pantaleo MA, Biasco G, Paterini P. Activated NF-κB in colorectal cancer: predictive or prognostic factor? J Clin Oncol. 2008;26:1388–1389.
Al Mulla F, MacKenzie EM. Differences in in vitro invasive capacity induced by differences in Ki-Ras protein mutations. J Pathol. 2001;195:549–556.
Al Mulla F, Milner-White EJ, Going JJ, Birnie GD. Structural differences between valine-12 and aspartate-12 Ras proteins may modify carcinoma aggression. J Pathol. 1999;187:433–438.
De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820.
Rohrer J, Wuertz BR, Ondrey F. Cigarette smoke condensate induces NF kappa-b activity and proangiogenic growth factors in aerodigestive cells. Laryngoscope. 2010;120:1609–1613.
Preciado D, Kuo E, Ashktorab S, et al. Cigarette smoke activates NF-kappaB-mediated TNF-α release from mouse middle ear cells. Laryngoscope. 2010;120:2508–2515.
Acknowledgments
We thank Drs Lin Chen (Department of Internal Medicine, Fujian Provincial Tumor Hospital) and Zhiwei Tang (Department of Immuno-Oncology Laboratory, Fujian Provincial Cancer Hospital) for helping with collection of the data. The project was supported by the Medical Innovation Foundation of Fujian Province (no. 2007-CXB-1).
Conflict of interest
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lin, G., Zheng, Xw., Li, C. et al. KRAS Mutation and NF-κB Activation Indicates Tolerance of Chemotherapy and Poor Prognosis in Colorectal Cancer. Dig Dis Sci 57, 2325–2333 (2012). https://doi.org/10.1007/s10620-012-2172-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2172-x