Abstract
Background
We previously demonstrated vagal neural pathways, specifically subdiaphragmatic afferent fibers, regulate expression of the intestinal sodium-glucose cotransporter SGLT1, the intestinal transporter responsible for absorption of dietary glucose. We hypothesized targeting this pathway could be a novel therapy for obesity. We therefore tested the impact of disrupting vagal signaling by total vagotomy or selective vagal de-afferentation on weight gain and fat content in diet-induced obese rats.
Methods
Male Sprague–Dawley rats (n = 5–8) underwent truncal vagotomy, selective vagal de-afferentation with capsaicin, or sham procedure. Animals were maintained for 11 months on a high-caloric Western diet. Abdominal visceral fat content was assessed by magnetic resonance imaging together with weight of fat pads at harvest. Glucose homeostasis was assessed by fasting blood glucose and HbA1C. Jejunal SGLT1 gene expression was assessed by qPCR and immunoblotting and function by glucose uptake in everted jejunal sleeves.
Results
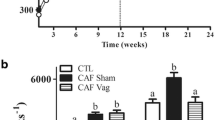
At 11-months, vagotomized rats weighed 19% less (P = 0.003) and de-afferented rats 7% less (P = 0.19) than shams. Vagotomized and de-afferented animals had 52% (P < 0.0001) and 18% reduction (P = 0.039) in visceral abdominal fat, respectively. There were no changes in blood glucose or glycemic indexes. SGLT1 mRNA, protein and function were unchanged across all cohorts at 11-months postoperatively.
Conclusions
Truncal vagotomy led to significant reductions in both diet-induced weight gain and visceral abdominal fat deposition. Vagal de-afferentation led to a more modest, but clinically and statistically significant, reduction in visceral abdominal fat. As increased visceral abdominal fat is associated with excess morbidity and mortality, vagal de-afferentation may be a useful adjunct in bariatric surgery.





Similar content being viewed by others
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi:10.1001/jama.292.14.1724.
Boss TJ, Peters J, Patti MG, Lustig RH, Kral JG. Laparoscopic truncal vagotomy for severe obesity: six month experience in 10 patients from a prospective, two-center study. Surg Obes Relat Dis. 2007;3:292.
Kral JG. Vagotomy for treatment of severe obesity. Lancet. 1978;1:307–308.
Kral JG. Effects of truncal vagotomy on body weight and hyperinsulinemia in morbid obesity. Am J Clin Nutr. 1980;33:416–419.
Camilleri M, Toouli J, Herrera MF, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143:723–731. doi:10.1016/j.surg.2008.03.015.
Stearns AT, Balakrishnan A, Rounds J, Rhoads DB, Ashley SW, Tavakkolizadeh A. Capsaicin-sensitive vagal afferents modulate posttranscriptional regulation of the rat Na+/glucose cotransporter SGLT1. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1078–G1083. doi:10.1152/ajpgi.00591.2007.
Martin MG, Lostao MP, Turk E, Lam J, Kreman M, Wright EM. Compound missense mutations in the sodium/d-glucose cotransporter result in trafficking defects. Gastroenterology. 1997;112:1206–1212.
Johnston KL, Clifford MN, Morgan LM. Possible role for apple juice phenolic, compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J Sci Food Agric. 2002;82:1800–1805.
Rossetti L, Smith D, Shulman GI, Papachristou D, Defronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515.
Melnyk A, Himms-Hagen J. Resistance to aging-associated obesity in capsaicin-desensitized rats one year after treatment. Obes Res. 1995;3:337–344.
Balakrishnan A, Stearns AT, Rounds J, et al. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1). Surgery. 2008;143:813–818. doi:10.1016/j.surg.2008.03.018.
Stearns AT, Balakrishnan A, Rhoads DB, Ashley SW, Tavakkolizadeh A. Diurnal expression of the rat intestinal sodium-glucose cotransporter 1 (SGLT1) is independent of local luminal factors. Surgery. 2009;145:294–302. doi:10.1016/j.surg.2008.11.004.
Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509.
Kral JG, Gortz L. Truncal vagotomy in morbid obesity. Int J Obes. 1981;5:431–435.
Kral JG. Vagotomy as a treatment for morbid obesity. Surg Clin North Am. 1979;59:1131–1138.
Kral JG, Gortz L, Hermansson G, Wallin GS. Gastroplasty for obesity: long-term weight loss improved by vagotomy. World J Surg. 1993;17:75–78.
Angrisani L, Cutolo PP, Ciciriello MB, et al. Laparoscopic adjustable gastric banding with truncal vagotomy versus laparoscopic adjustable gastric banding alone: interim results of a prospective randomized trial. Surg Obes Relat Dis. 2009;5:435–438. doi:10.1016/j.soard.2008.08.024.
Ferrari B, Arnold M, Carr RD, et al. Subdiaphragmatic vagal deafferentation affects body weight gain and glucose metabolism in obese male Zucker (fa/fa) rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1027–R1034. doi:10.1152/ajpregu.00736.2004.
Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham heart study. Circulation. 2007;116:39–48. doi:10.1161/circulationaha.106.675355.
Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284:E1065–E1071. doi:10.1152/ajpendo.00442.200200442.2002.
Banerji MA, Buckley MC, Chaiken RL, Gordon D, Lebovitz HE, Kral JG. Liver fat, serum triglycerides and visceral adipose tissue in insulin-sensitive and insulin-resistant black men with NIDDM. Int J Obes Relat Metab Disord. 1995;19:846–850.
Diamant M, Lamb HJ, van de Ree MA, et al. The association between abdominal visceral fat and carotid stiffness is mediated by circulating inflammatory markers in uncomplicated type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1495–1501. doi:10.1210/jc.2004-1579.
Despres JP, Lemieux S, Lamarche B, et al. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord. 1995;19:S76–S86.
Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring). 2006;14:336–341. doi:10.1038/oby.2006.43.
Carroll JF, Franks SF, Smith AB, Phelps DR. Visceral adipose tissue loss and insulin resistance 6 months after laparoscopic gastric banding surgery: a preliminary study. Obes Surg. 2009;19:47–55. doi:10.1007/s11695-008-9642-4.
Powley TL, Chi MM, Baronowsky EA, Phillips RJ. Gastrointestinal tract innervation of the mouse: afferent regeneration and meal patterning after vagotomy. Am J Physiol Regul Integr Comp Physiol. 2005;289:R563–R574. doi:10.1152/ajpregu.00167.2005.
Phillips RJ, Baronowsky EA, Powley TL. Long-term regeneration of abdominal vagus: efferents fail while afferents succeed. J Comp Neurol. 2003;455:222–237. doi:10.1002/cne.10470.
Phillips RJ, Baronowsky EA, Powley TL. Regenerating vagal afferents reinnervate gastrointestinal tract smooth muscle of the rat. J Comp Neurol. 2000;421:325–346. doi:10.1002/(SICI)1096-9861(20000605)421:3<325:AID-CNE3>3.0.CO;2-9.
Ritter RC, Ladenheim EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol. 1985;248:R501–R504.
Mordes JP, Lozy ME, Herrera MG, Silen W. Effects of vagotomy with and without pyloroplasty on weight and food-intake in rats. Am J Physiol. 1979;236:R61–R66.
Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi:10.1038/nature05484.
Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi:10.1126/science.1109951.
Leung FW, Go VL, Scremin OU, et al. Pilot studies to demonstrate that intestinal mucosal afferent nerves are functionally linked to visceral adipose tissue. Dig Dis Sci. 2007;52:2695–2702. doi:10.1007/s10620-006-9645-8.
Pardo JV, Sheikh SA, Kuskowski MA, et al. Weight loss during chronic, cervical vagus nerve stimulation in depressed patients with obesity: an observation. Int J Obes (Lond). 2007;31:1756–1759. doi:10.1038/sj.ijo.0803666
Esposito G, Faelli A, Tosco M, Orsenigo MN, Battistessa R. Age-related changes in rat intestinal transport of d-glucose, sodium, and water. Am J Physiol. 1985;249:G328–G334.
Vincenzini MT, Iantomasi T, Stio M, et al. Glucose transport during ageing by human intestinal brush-border membrane vesicles. Mech Ageing Dev. 1989;48:33–41.
Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol. 2009;297:G950–G957.
Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241–G248. doi:10.1152/ajpgi.00310.2001.
Gram DX, Ahren B, Nagy I, et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213–223. doi:10.1111/j.1460-9568.2006.05261.x.
Gram DX, Hansen AJ, Wilken M, et al. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur J Endocrinol. 2005;153:963–969. doi:10.1530/eje.1.02046.
Gram DX, Hansen AJ, Deacon CF, et al. Sensory nerve desensitization by resiniferatoxin improves glucose tolerance and increases insulin secretion in Zucker Diabetic Fatty rats and is associated with reduced plasma activity of dipeptidyl peptidase IV. Eur J Pharmacol. 2005;509:211–217. doi:10.1016/j.ejphar.2004.12.039.
Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–2262.
Acknowledgments
Funding sources include: Harvard Clinical Nutrition Center Grant P30-DK040561 (AT); Berkeley Fellowship and George Herbert Hunt Travelling Fellowship (ATS); and Nutricia Foundation Fellowship (AB). We would like to thank Dr Sharon Peled, and Dr Clare Tempany-Afdhal for technical advice and support with magnetic resonance image acquisition, and Jan Rounds for invaluable managerial support.
Conflict of interest
No authors have any conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stearns, A.T., Balakrishnan, A., Radmanesh, A. et al. Relative Contributions of Afferent Vagal Fibers to Resistance to Diet-Induced Obesity. Dig Dis Sci 57, 1281–1290 (2012). https://doi.org/10.1007/s10620-011-1968-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1968-4