Abstract
Background
The etiology of inflammatory bowel diseases (IBD) is largely unknown, but appears to be perpetuated by uncontrolled responses to antigenic components of the endogenous flora. Tolerance to antigenic stimulation can be achieved by exposure to a given antigen in high amounts (high dose tolerance). Colitis induced by feeding of Dextran Sodium Sulfate (DSS) is an often-used animal model mimicking clinical and histological features of human IBD.
Aims
We investigated whether treatment with high doses of endogenous bacterial components can affect the response to these antigenic components and thus impact the course of the inflammatory response induced by DSS.
Methods
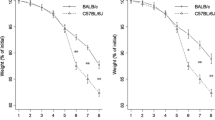
129/SvEv mice were injected intravenously in the tail vein with lysates prepared from fecal material of conventionally-raised mice. Control mice received a solution of bacterial antigen-free lysates prepared from fecal material of germ-free mice. Seven days later, colitis was induced in these mice by introducing DSS (3.5%) in the drinking water for 5 days. Onset and course of the inflammatory response was monitored by assessment of weight loss. Mice were sacrificed at day 7 post colitis induction and tested for histopathologic injury, intestinal cytokine release, and systemic response to bacterial antigens.
Results
Intravenous injection with fecal lysates reduced intestinal and antigen-stimulated systemic pro-inflammatory cytokine release and prevented DSS-induced weight loss and intestinal injury.
Conclusion
Pretreatment with high amount of endogenous bacterial components has a profound tolerogenic effect on the systemic and mucosal immune responses resulting in reduced intestinal inflammation and abrogates colitis-induced weight loss.





Similar content being viewed by others
References
Shroff KE, Meslin K, Cebra JJ. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913.
Sydora BC, MacFarlane SM, Lupicki M, et al. An imbalance in mucosal cytokine profile causes transient intestinal inflammation following an animal’s first exposure to faecal bacteria and antigens. Clin Exp Immunol. 2010;161:187–196.
Jump RL, Levine AD. Mechanisms of natural tolerance in the intestine: implications for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:462–478.
Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280.
Jenkins MK, Pardoll DM, Mizuguchi J, et al. T-cell unresponsiveness in vivo and in vitro: fine specificity of induction and molecular characterization of the unresponsive state. Immunol Rev. 1987;95:113–135.
Sakaguchi S. Immunologic tolerance maintained by regulatory T cells: implications for autoimmunity, tumor immunity and transplantation tolerance. Vox Sang. 2002;83:151–153.
Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19.
Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726.
Sydora BC, Macfarlane SM, Doyle JS, et al. Neonatal exposure to fecal antigens reduces intestinal inflammation. Inflamm Bowel Dis. 2010;17:899–906.
Verdu EF, Bercik P, Cukrowska B, et al. Oral administration of antigens from intestinal flora anaerobic bacteria reduces the severity of experimental acute colitis in BALB/c mice. Clin Exp Immunol. 2000;120:46–50.
Konrad A, Mahler M, Flogerzi B, et al. Amelioration of murine colitis by feeding a solution of lysed Escherichia coli. Scand J Gastroenterol. 2003;38:172–179.
Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546.
Yan Y, Kolachala V, Dalmasso G, et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS ONE. 2009;4:6073.
Kitajima S, Takuma S, Morimoto M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Anim. 2000;49:9–15.
Dieleman LA, Hoentjen F, Qian BF, et al. Reduced ratio of protective versus proinflammatory cytokine responses to commensal bacteria in HLA-B27 transgenic rats. Clin Exp Immunol. 2004;136:30–39.
Sydora BC, Tavernini MM, Wessler A, et al. Lack of interleukin-10 leads to intestinal inflammation, independent of the time at which luminal microbial colonization occurs. Inflamm Bowel Dis. 2003;9:87–97.
Madsen KL, Doyle JS, Jewell LD, et al. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114.
Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352.
Egger B, Bajaj-Elliott M, MacDonald TT, et al. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–248.
Duchmann R, Kaiser I, Hermann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol. 1995;102:448–455.
Sakaguchi S. Naturally arising CD4 + regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562.
Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302.
Uhlig HH, Powrie F. The role of mucosal T lymphocytes in regulating intestinal inflammation. Springer Semin Immunopathol. 2005;27:167–180.
Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4 + CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878.
O’Mahony C, van der Kleij H, Bienenstock J, et al. Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1118–R1126.
Kitajima S, Morimoto M, Sagara E, et al. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim. 2001;50:387–395.
Weigle WO. Immunological unresponsiveness. Adv Immunol. 1973;16:61–122.
Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256.
Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228.
Liblau RS, Tisch R, Shokat K, et al. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci USA. 1996;93:3031–3036.
Burstein HJ, Abbas AK. In vivo role of interleukin 4 in T cell tolerance induced by aqueous protein antigen. J Exp Med. 1993;177:457–463.
Rizzo LV, Morawetz RA, Miller-Rivero NE, et al. IL-4 and IL-10 are both required for the induction of oral tolerance. J Immunol. 1999;162:2613–2622.
Chen Y, Inobe J, Marks R, et al. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180.
Troy AE, Zaph C, Du Y, et al. IL-27 regulates homeostasis of the intestinal CD4 + effector T cell pool and limits intestinal inflammation in a murine model of colitis. J Immunol. 2009;183:2037–2044.
Veltkamp C, Ruhwald R, Giesem T, et al. CD4 + CD25 + cell depletion from the normal CD4 + T cell pool prevents tolerance toward the intestinal flora and leads to chronic colitis in immunodeficient mice. Inflamm Bowel Dis. 2006;12:437–446.
Ohkawara T, Nishihira J, Takeda H, et al. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123:256–270.
Horino J, Fujimoto M, Terabe F, et al. Suppressor of cytokine signaling-1 ameliorates dextran sulfate sodium-induced colitis in mice. Int Immunol. 2008;20:753–762.
Dieleman LA, Ridwan BU, Tennyson GS, et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652.
Kim TW, Seo JN, Suh YH, et al. Involvement of lymphocytes in dextran sulfate sodium-induced experimental colitis. World J Gastroenterol. 2006;12:302–305.
Moskophidis D, Lechner F, Pircher H, et al. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761.
Oxenius A, Zinkernagel RM, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4 + and CD8 + T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sydora, B.C., Albert, E.J., Foshaug, R.R. et al. Intravenous Injection of Endogenous Microbial Components Abrogates DSS-Induced Colitis. Dig Dis Sci 57, 345–354 (2012). https://doi.org/10.1007/s10620-011-1878-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1878-5