Abstract
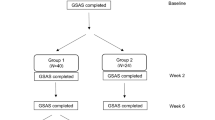
The pathogenesis of patient dissatisfaction following involuntary therapeutic substitutions involving proton pump inhibitors (PPIs) is poorly understood. The aim of this study was to describe the patient population experiencing therapeutic failure and investigate whether failure was related to individual differences in response to the different PPIs. Treatment failures in a lansoprazole-rabeprazole therapeutic substitution program were compared to switch successes. A subgroup was randomized in a double-blind, double-dummy, crossover study to four 2-week periods of lansoprazole-rabeprazole-lansoprazole-rabeprazole or vice versa. Measures included overall rating of gastrointestinal reflux disease (GERD) symptoms for the past week as well as the frequency and distress scales of the GERD Symptom Assessment Scale. One hundred fifteen nonresponders were compared with 54 successful responders. Nonresponders consisted primarily of patients with GERD (74%, vs. 44% of responders; P = 0.005) who had undergone upper gastrointestinal endoscopy (50%, vs. 31% of responders; P = 0.02). Twelve patients completed the randomized treatment study. The interrater κ coefficient for responder status was estimated to be 0.80 for lansoprazole and 0.21 for rabeprazole. The majority of PPI nonresponders had a clinical diagnosis of GERD and were receiving ≥40 mg of rabeprazole daily. This pilot study provides new insights into the design of subsequent studies of nonresponders in PPI therapeutic substitution programs.
Similar content being viewed by others
References
Furberg CD, Herrington DM, Psaty BM (1999) Are drugs within a class interchangeable? Lancet 354:1202–1204
Furberg CD, Psaty BM (2003) Should evidence-based proof of drug efficacy be extrapolated to a “class of agents”? Circulation 108:2608–2610
Kereiakes DJ, Willerson JT (2003) Therapeutic substitution: guilty until proven innocent. Circulation 108:2611–2612
Gerson LB, Hatton BN, Ryono R, Jones W, Pulliam G, Sampliner RE, Triadafilopoulos G, Fass R (2000) Clinical and fiscal impact of lansoprazole intolerance in veterans with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 14:397–406
Nelson WW, Vermeulen LC, Geurkink EA, Ehlert DA, Reichelderfer M (2000) Clinical and humanistic outcomes in patients with gastroesophageal reflux disease converted from omeprazole to lansoprazole. Arch Intern Med 160:2491–2496
Amidon PB, Jankovich R, Stoukides CA, Kaul AF (2000) Proton pump inhibitor therapy: preliminary results of a therapeutic interchange program. Am J Manag Care 6:593–601
Condra LJ, Morreale AP, Stolley SN, Marcus D (1999) Assessment of patient satisfaction with a formulary switch from omeprazole to lansoprazole in gastroesophageal reflux disease maintenance therapy. Am J Manag Care 5:631–638
Baber N, Halliday LD, Van Den Heuvel WJ, Walker RW, Sibeon R, Keenan JP, Littler T, Orme ML (1979) Indomethacin in rheumatoid arthritis: clinical effects, pharmacokinetics, and platelet studies in responders and nonresponders. Ann Rheum Dis 38:128–136
Brooks PM, Day RO (1991) Nonsteroidal antiinflammatory drugs—differences and similarities. N Engl J Med 324:1716–1725
Day RO, Graham GG, Williams KM, Brooks PM (1988) Variability in response to NSAIDs. Fact or fiction? Drugs 36:643–651
Huskisson EC, Woolf DL, Balme HW, Scott J, Franklin S (1976) Four new anti-inflammatory drugs: responses and variations. Br Med J 1:1048–1049
Huskisson EC (1977) Correlation of experimental studies and human responses to anti-rheumatic drugs. Acta Clin Belg 32:223–229
Walker JS, Sheather-Reid RB, Carmody JJ, Vial JH, Day RO (1997) Nonsteroidal antiinflammatory drugs in rheumatoid arthritis and osteoarthritis: support for the concept of “responders” and “nonresponders.” Arthritis Rheum 40:1944–1954
Day RO, Brooks PM (1987) Variations in response to non-steroidal anti-inflammatory drugs. Br J Clin Pharmacol 23:655–658
Johnson M, Guilford S, Libretto SE (2002) Patients have treatment preferences: a multicentre, double-blind, crossover study comparing rabeprazole and omeprazole. Curr Med Res Opin 18:303–310
Rothman M, Farup C, Stewart W, Helbers L, Zeldis J (2001) Symptoms associated with gastroesophageal reflux disease: development of a questionnaire for use in clinical trials. Dig Dis Sci 46:1540–1549
Raisch DW, Klaurens LM, Hayden C, Malagon I, Pulliam G, Fass R (2001) Impact of a formulary change in proton pump inhibitors on health care costs and patients’ symptoms. Dig Dis Sci 46:1533–1539
Sodorff MM, Galt KA, Galt MA, Turner PD, Lambrecht JE (2002) Patient perceptions of a proton pump inhibitor therapeutic interchange program across the continuum of care. Pharmacotherapy 22:500–512
Kahrilas PJ, Falk GW, Johnson DA, Schmitt C, Collins DW, Whipple J, D’Amico D, Hamelin B, Joelsson B (2000) Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: a randomized controlled trial. The Esomeprazole Study Investigators. Aliment Pharmacol Ther 14:1249–1258
Acknowledgments
This was an investigator-initiated study supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and by Public Health Service Grant DK56338, which funds the Texas Gulf Coast Digestive Diseases Center. Small amounts of funds were made available to support the IRB costs and drugs and matching placebos were supplied by PriCara, Unit of Ortho-McNeil, Inc., and Eisai Inc. and TAP. None of the authors who were not employees of TAP were paid consultants for TAP or other makers of proton pump inhibitors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, M., Malladi, V., Agha, A. et al. Failures in a Proton Pump Inhibitor Therapeutic Substitution Program: Lessons Learned. Dig Dis Sci 52, 2813–2820 (2007). https://doi.org/10.1007/s10620-007-9811-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9811-7