Abstract
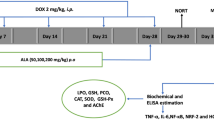
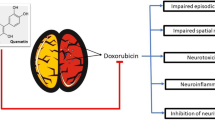
Cognitive dysfunction by chemotherapy compromises the quality of life in cancer patients. Tea polyphenols are known chemopreventive agents. The present study was designed to evaluate the neuroprotective potential of (+) catechin hydrate (catechin), a tea polyphenol, in IMR-32 neuroblastoma cells in vitro and alleviation of episodic memory deficit in Wistar rats in vivo against a widely used chemotherapeutic agent, Doxorubicin (DOX). In vitro, neuroprotective studies were assessed in undifferentiated IMR-32 cells using percentage viability and in differentiated cells by neurite length. These studies showed catechin increased percentage viability of undifferentiated IMR-32 cells. Catechin pretreatment also showed an increase in neurite length of differentiated cells. In vivo neuroprotection of catechin was evaluated using novel object recognition task in time-induced memory deficit model at 50, 100 and 200 mg/kg dose and DOX-induced memory deficit models at 100 mg/kg dose. The latter model was developed by injection of DOX (2.5 mg/kg, i.p.) in 10 cycles over 50 days in Wistar rats. Catechin showed a significant reversal of time-induced memory deficit in a dose-dependent manner and prevention of DOX-induced memory deficit at 100 mg/kg. In addition, catechin treatment showed a significant decrease in oxidative stress, acetylcholine esterase and neuroinflammation in the hippocampus and cerebral cortex in DOX-induced toxicity model. Hence, catechin may be a potential adjuvant therapy for the amelioration of DOX-induced cognitive impairment which may improve the quality of life of cancer survivors. This improvement might be due to the elevation of antioxidant defense, prevention of neuroinflammation and inhibition of acetylcholine esterase enzyme.







Similar content being viewed by others
References
Abd El-Aziz TA, Mohamed RH, Pasha HF, Abdel-Aziz HR (2012) Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med 12:233–240
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahles TA, Saykin AJ (2002) Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer Suppl 3:S84–S90
Ahles TA, Saykin AJ (2007) Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 7:192–201
Ahmed ME, Khan MM, Javed H, Vaibhav K, Khan A, Tabassum R, Ashafaq M, Islam F, Safhi MM, Islam F (2013) Amelioration of cognitive impairment and neurodegeneration by catechin hydrate in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Neurochem Int 62:492–501. doi:10.1016/j.neuint.2013.02.006
Aluise CD, Sultana R, Tangpong J, Vore M, Clair DS, Moscow JA, Butterfield DA (2010a) Chemo brain clinical breast cancer (chemo fog) as a potential side effect of doxorubicin administration: role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction, Chemo Fog. Springer, Berlin, pp 147–156
Aluise CD, Sultana R, Tangpong J, Vore M, St Clair D, Moscow JA, Butterfield DA (2010b) Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv Exp Med Biol 678:147–156
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Asensio-López MC, Soler F, Sánchez-Más J, Pascual-Figal D, Fernández-Belda F, Lax A (2016) Early oxidative damage induced by doxorubicin: source of production, protection by GKT137831 and effect on Ca2+ transporters in HL-1 cardiomyocytes. Arch Biochem Biophys 594:26–36
Asensio-López MC, Soler F, Pascual-Figal D, Fernández-Belda F, Lax A (2017) Doxorubicin-induced oxidative stress: the protective effect of nicorandil on HL-1 cardiomyocytes. PLoS ONE 12:e0172803
Assuncao M, Andrade JP (2015) Protective action of green tea catechins in neuronal mitochondria during aging. Front Biosci (Landmark Ed) 20:247–262
Boykoff N, Moieni M, Subramanian SK (2009) Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv 3:223–232
Braidy N, Grant R, Adams S, Guillemin GJ (2010) Neuroprotective effects of naturally occurring polyphenols on quinolinic acid-induced excitotoxicity in human neurons. FEBS J 277:368–382. doi:10.1111/j.1742-4658.2009.07487.x
Bredt DS, Snyder SH (1994) Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63:175–195
Cheng T, Liu J, Ren J, Huang F, Ou H, Ding Y, Zhang Y, Ma R, An Y, Liu J, Shi L (2016) Green tea catechin-based complex micelles combined with doxorubicin to overcome cardiotoxicity and multidrug resistance. Theranostics 6:1277–1292
Christen Y (2000) Oxidative stress and Alzheimer disease. Am J Clin Nutr 71:621S–629S
Deavall DG, Martin EA, Horner JM, Roberts R (2012) Drug-induced oxidative stress and toxicity. J Toxicol 2012:13
Dholwani K, Saluja A, Gupta A, Shah D (2008) A review on plant-derived natural products and their analogs with anti-tumor activity. Indian J Pharmacol 40:49–58. doi:10.4103/0253-7613.41038
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fawcett JR, Bordayo EZ, Jackson K, Liu H, Peterson J, Svitak A, Frey Ii WH (2002) Inactivation of the human brain muscarinic acetylcholine receptor by oxidative damage catalyzed by a low molecular weight endogenous inhibitor from Alzheimer’s brain is prevented by pyrophosphate analogs, bioflavonoids and other antioxidants. Brain Res 950:10–20
Goodman LS (1996) Goodman and Gilman’s the pharmacological basis of therapeutics. McGraw-Hill, New York
Graff G, Gamache DA, Brady MT, Spellman JM, Yanni JM (1998) Improved myeloperoxidase assay for quantitation of neutrophil influx in a rat model of endotoxin-induced uveitis. J Pharmacol Toxicol Methods 39:169–178
Grandhi RV, Gourishetti K, Kishore A, Nandakumar K (2016) Assessment of female rats for studying episodic memory and its deficit associated with doxorubicin-induced chemobrain. Clin Exp Pharmacol Physiol 43:644–646. doi:10.1111/1440-1681.12568
Higdon JV, Frei B (2003) Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 43:89–143
Hu ML (1994) Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol 233:380–385
John J, Nampoothiri M, Kumar N, Mudgal J, Nampurath GK, Chamallamudi MR (2015) Sesamol, a lipid lowering agent, ameliorates aluminium chloride induced behavioral and biochemical alterations in rats. Pharmacogn Mag 11:327–336
Kataria H, Wadhwa R, Kaul SC, Kaur G (2012) Water extract from the leaves of Withania somnifera protect RA differentiated C6 and IMR-32 cells against glutamate-induced excitotoxicity. PLoS ONE 7:e37080
Khan KA, Kumar N, Nayak PG, Nampoothiri M, Shenoy RR, Krishnadas N, Rao CM, Mudgal J (2013) Impact of caffeic acid on aluminium chloride-induced dementia in rats. J Pharm Pharmacol 65:1745–1752. doi:10.1111/jphp.12126
Kim DS, Kim DS, Oppel MN (2002) Shogaols from Zingiber officinale protect IMR32 human neuroblastoma and normal human umbilical vein endothelial cells from beta-amyloid(25–35) insult. Planta Med 68:375–376
Konings A, Drijver E (1979) Radiation effects on membranes: I. Vitamin E deficiency and lipid peroxidation. Radiat Res 80:494–501
Kumar N, Rai A, Reddy ND, Raj PV, Jain P, Deshpande P, Mathew G, Kutty NG, Udupa N, Rao CM (2014) Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol Rep 66:788–798. doi:10.1016/j.pharep.2014.04.007
Manchon JFM, Dabaghian Y, Uzor NE, Kesler SR, Wefel JS, Tsvetkov AS (2016) Levetiracetam mitigates doxorubicin-induced DNA and synaptic damage in neurons. Sci Rep 6:25705
McIntosh LJ, Sapolsky RM (1996) Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp Neurol 141:201–206
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Mohamed RH, Karam RA, Amer MG (2011) Epicatechin attenuates doxorubicin-induced brain toxicity: critical role of TNF-alpha, iNOS and NF-kappaB. Brain Res Bull 86:22–28
Nampoothiri M, Reddy ND, John J, Kumar N, Kutty Nampurath G, Rao Chamallamudi M (2014) Insulin blocks glutamate-induced neurotoxicity in differentiated SH-SY5Y neuronal cells. Behav Neurol 2014:674164
Nampoothiri M, John J, Kumar N, Mudgal J, Nampurath GK, Chamallamudi MR (2015) Modulatory role of simvastatin against aluminium chloride-induced behavioural and biochemical changes in rats. Behav Neurol 2015:210169
Nath S, Bachani M, Harshavardhana D, Steiner JP (2012) Catechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathway. J Neurovirol 18:445–455
Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL (2012) Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52:1213–1225
Rang HP (2007) Rang and Dale’s pharmacology. Churchill Livingstone, Philadelphia, pp 672–674
Reddy ND, Shoja MH, Jayashree BS, Nayak PG, Kumar N, Prasad VG, Pai KS, Rao CM (2015) In vitro and in vivo evaluation of novel cinnamyl sulfonamide hydroxamate derivative against colon adenocarcinoma. Chem Biol Interact 233:81–94
Schimmel KJ, Richel DJ, van den Brink RB, Guchelaar HJ (2004) Cardiotoxicity of cytotoxic drugs. Cancer Treat Rev 30:181–191
Shi Y, Ma IT, Patel RH, Shang X, Chen Z, Zhao Y, Cheng J, Fan Y, Rojas Y, Barbieri E, Chen Z, Yu Y, Jin J, Kim ES, Shohet JM, Vasudevan SA, Yang J (2015) NSC-87877 inhibits DUSP26 function in neuroblastoma resulting in p53-mediated apoptosis. Cell Death Dis 6:e1841
Simon L, Srinivasan KK, Kumar N, Reddy ND, Biswas S, Rao CM, Moorkoth S (2016) Selected novel 5′-amino-2′-hydroxy-1,3-diaryl-2-propen-1-ones arrest cell cycle of Hct-116 in G0/G1 phase. EXCLI J 15:21–32
Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, Severin SE, Uhl R, Kock M, Geiger KD (2004) Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer 109:759–767
Suganuma M, Saha A, Fujiki H (2011) New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci 102:317–323
Swamy AV, Wangikar U, Koti B, Thippeswamy A, Ronad P, Manjula D (2011) Cardioprotective effect of ascorbic acid on doxorubicin-induced myocardial toxicity in rats. Indian J Pharmacol 43:507
Tabakman R, Lazarovici P, Kohen R (2002) Neuroprotective effects of carnosine and homocarnosine on pheochromocytoma PC12 cells exposed to ischemia. J Neurosci Res 68:463–469
Teixeira MD, Souza CM, Menezes AP, Carmo MR, Fonteles AA, Gurgel JP, Lima FA, Viana GS, Andrade GM (2013) Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol Biochem Behav 110:1–7
Walle T (2007) Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Seminars in cancer biology. Elsevier, New York, pp 354–362
Yazawa K, Kihara T, Shen H, Shimmyo Y, Niidome T, Sugimoto H (2006) Distinct mechanisms underlie distinct polyphenol-induced neuroprotection. FEBS Lett 580:6623–6628
Acknowledgements
We thank AICTE (RPS Scheme—Grant No. 20/AICTE/RIFD/RPS (POLICY-1)64/2013-14) and Manipal University for infrastructural support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheruku, S.P., Ramalingayya, G.V., Chamallamudi, M.R. et al. Catechin ameliorates doxorubicin-induced neuronal cytotoxicity in in vitro and episodic memory deficit in in vivo in Wistar rats. Cytotechnology 70, 245–259 (2018). https://doi.org/10.1007/s10616-017-0138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-017-0138-8