Abstract
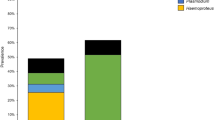
Our current understanding of migration routes of many birds is limited and researchers have employed various methods to determine migratory patterns. Recently, parasites have been used to track migratory birds. The objective of this study was to determine whether haemosporidian parasite lineages detect significant geographic structure in common yellowthroats (Geothlypis trichas). We examined liver tissue or blood from 552 birds sampled from multiple locations throughout the continental United States, southern Canada, and the Bahamas. We found a 52.7% overall prevalence of haematozoan infection. We identified 86.1% of these infections to genus: 81% were Plasmodium; 5% were Haemoproteus; and 0.1% were Leucocytozoon. There were significant differences in the prevalence of different parasite genera among regions (χ2 = 36.82, P < 0.0001) and in the proportion of Plasmodium infections versus other parasites among regions (χ2 = 35.52, P < 0.0001). Sequence information identified three Haemoproteus lineages, two Leucocytozoon lineages, and thirteen Plasmodium lineages. Due to the low number of Haemoproteus and Leucocytozoon, only Plasmodium lineages were used in the geographic comparison of lineages. Six Plasmodium lineages were found in eight or more birds and the prevalence of these varied significantly among regions (χ2 = 172.33, P < 0.0001). Additionally, 45 juvenile birds were sampled to determine what parasites could be obtained in the breeding grounds and we found only one lineage. In conclusion, parasite lineages show some geographic structure, with some lineages being more geographically specific than others, but are not useful for determining migratory connectivity in this species.



Similar content being viewed by others
References
Alavi Y, Arai M, Mendoza J et al (2003) The dynamics of interactions between Plasmodium and the mosquito: a study of the infectivity of Plasmodium berghei and Plasmodium gallinaceum, and their transmission by Anopheles stephensi, Anopheles gambiae and Aedes aegypti. Int J Parasitol 33:933–943
Atkinson CT, van Riper C III (1991) Pathogenicity and epizootiology of avian hematozoa: plasmodium, leucocytozoon, and haemoproteus. In: Loye JE, Zuk M (eds) Bird-parasite interactions. Oxford University Press, Oxford, pp 19–48
Atkinson C, Forrester D, Greiner E (1988) Epizootiology of Haemoproteus meleagridis (Protozoa: Haemosporina) in Florida: seasonal transmission and vector abundance. J Med Entomol 25:45–51
Ball RM Jr, Avise J (1992) Mitochondrial DNA phylogeographic differentiation among avian populations and the evolutionary significance of subspecies. Auk 109:626–636
Ballard G, Geupei G, Nur N et al (2003) Long-term declines and decadal patterns in population trends of songbirds in western North America, 1979–1999. Condor 105:737–755
Beadell J, Fleischer R (2005) A restriction enzyme-based assay to distinguish between avian hemosporidians. J Parasitol 91:683–685
Beadell J, Gering E, Austin J et al (2004) Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Mol Ecol 13:3829–3844
Beadell JS, Ishtiaq F, Covas R et al (2006) Global phylogeographic limits in Hawaii’s avian malaria. Proc R Soc Lond B 273:2935–2944
Bensch S, Åkesson S (2003) Temporal and spatial variation of hematozoans in Scandinavian Willow Warblers. J Parasitol 89:388–391
Bensch S, Stjernman M, Hasselquist D et al (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc R Soc Lond B 267:1583–1589
Bensch S, Perez-Tris J, Waldenström J et al (2004) Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? Evolution 58:1617–1621
Bensch S, Waldenström J, Jonzén N et al (2007) Temporal dynamics and diversity of avian malaria parasites in a single host species. J Anim Ecol 76:112–122
Biek R, Drummond A, Poss M (2006) A virus reveals populations structure and recent demographic history of its carnivore host. Science 311:538–541
Blouin M, Yowell C, Courtney C et al (1995) Host movement and the genetic structure of populations of parasitic nematodes. Genetics 141:1007–1014
Booth C, Elliott P (2003) Hematological responses to hematozoa in North America and neotropical songbirds. Comp Biochem Physiol 133:451–467
Chamberlain CP, Blum JD, Holmes RT et al (1997) The use of stable isotope tracers for identifying populations of migratory birds. Oecologia 109:132–141
Cosgrove CL, Knowles SCL, Day KP et al (2006) No evidence for avian malaria infection during the nestling phase in a passerine bird. J Parasitol 92:1302–1304
Criscione C, Blouin M (2004) Life cycles shape parasite evolution: comparative population genetics of salmon trematodes. Evolution 58:198–202
Davidar P, Morton E (1993) Living with parasites: prevalence of a blood parasite and its effect on survivorship in the Purple Martin. Auk 110:109–116
Dumbacher J, Pratt T, Fleischer R (2003) Phylogeny of the owlet-nightjars (Aves: Aegothelidae) based on mitochondrial DNA sequence. Mol Phylogenet Evol 29:540–549
Durrant KL, Beadell JS, Ishtiaq F et al (2006) Avian Hematozoa in South America: a comparison of temperate and tropical zones. Ornithol Monogr 60:98–111
Escalante A, Freeland D, Collins W et al (1998) The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc R Soc Lond B 95:8124–8129
Fallon SM, Ricklefs RE, Swanson BL, Bermingham E (2003) Detecting avian malaria: an improved polymerase chain reaction diagnostic. J Parasitol 89:1044–1047
Fallon S, Fleischer R, Graves G (2006) Malarial parasites as geographical markers in migratory birds? Biol Lett 2:213–216
Falush D, Wirth T, Linz B et al (2003) Traces of human migrations in helicobacter pylori populations. Science 299:1582–1585
Greiner E, Bennett G, White E et al (1975) Distribution of the avian hematozoa of North America. Can J Zool 53:1762–1787
Gylfe A, Bergström S, Lundström J et al (2000) Reactivation of Borrelia infection in birds. Nature 403:724–725
Haig SM, Gratto-Trevor CL, Mullins TD et al (1997) Population identification of western hemisphere shorebirds throughout the annual cycle. Mol Ecol 6:413–427
Hasselquist D, Östman Ö, Waldenström J et al (2007) Temporal patterns of occurrence and transmission of the blood parasite Haemoproteus payevskyi in the great reed warbler Acrocephalus arundinaceus. J Ornithol. Early Online Publishing
Hellgren O, Waldenström J, Peréz-Tris J et al (2007) Detecting shifts of transmission in avian blood parasites – a phylogenetic approach. Mol Ecol 16:1281–1290
Hobson K, Wassenaar L (1997) Linking breeding and wintering grounds of Neotropical migrant songbirds using stable isotopic analysis of feathers. Oecologia 109:142–148
Hobson K, McFarland K, Wassenaar L et al (2001) Linking breeding and wintering grounds of Bicknell’s thrushes using stable isotope analyses of feathers. Auk 118:16–23
Hobson K, Aubry Y, Wassenaar L (2004) Migratory connectivity in Bicknell’s thrush: locating missing populations with hydrogen isotopes. Condor 106:905–909
Hubalek Z (2004) An annotated checklist of pathogenic microorganisms associated with migratory birds. J Wildl Dis 40:639–659
Ishtiaq F, Beadell JS, Baker AJ et al (2006) Prevalence and evolutionary relationships of haemotozoan parasites in native versus introduced populations of common myna Acridotheres tristis. Proc R Soc B: Biol Sci 273:587–594
Jarvi S, Atkinson CT, Fleischer RC (2001) Immunogenetics and resistance to avian malaria in Hawaiian honeycreepers (Drepanidinae). Stud Avian Biol 22:254–263
Kimura M, Clegg SM, Lovette IJ et al. (2002) Phylogeographical approaches to assessing demographic connectivity between breeding and overwintering regions in the Nearctic-Neotropical warbler (Wilsonia pusilla). Mol Ecol 11:1605–1616
Klei T, DeGiusti D (1975) Seasonal occurrence of Haemoproteus columbae Kruse and its vector Pseudolynchia canariensis Bequaert. J Wildl Dis 11:130–134
Latta SC, Baltz ME (1997) Population limitation in neotropical migratory birds: comments on Rappole and McDonald (1994). Auk 114:754–762
Lovette IJ, Clegg SM, Smith TB (2004) Limited utility of mtDNA markers for determining connectivity among breeding and overwintering locations in three neotropical migrant birds. Conserv Biol 18:156–166
Marra P, Hobson K, Holmes RT (1998) Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282:1884–1886
Martell MS, Henny C, Nye P et al (2001) Fall migration routes, timing, and wintering sites of North American Ospreys as determined by satellite telemetry. Condor 103:715–724
Merino S, Potti J (1995) High prevalence of Hematozoa in Nestlings of a Passerine Species, the Pied Flycatcher (Ficedula hypoleuca). Auk 112:1041–1043
Merino S, Potti J (1995) High prevalence of hematozoa in nestlings of a passerine species, the Pied Flycatcher (Ficedula hypoleuca). Auk 112:1041–1043
Milot E, Gibbs HL, Hobson K (2000) Phylogeography and genetic structure of northern populations of the yellow warbler (Dendroica petechia). Mol Ecol 9:667–681
Paul R, Nu VAT, Krettli AU et al (2002) Interspecific competition during transmission of two sympatric malaria parasite species to the mosquito vector. Proc R Soc Lond B 269:2551–2557
Pérez-Tris J, Bensch S (2005) Dispersal increases local transmission of avian malarial parasites. Ecol Lett 8:838–845
Peterson AT, Vieglais DA, Andreasen JK (2003) Migratory birds modeled as critical transport agents for West Nile virus in North America. Vector-Borne Zoonotic Dis 3:27–37
Pitra C, D’Aloia MA, Lieckfeldt D et al (2004) Genetic variation across the current range of the Asian houbara bustard (Chlamydotis undulate macqueeni), Conserv Genet 5:205–215
Richard FA, Sehgal RNM, Jones HI et al (2002) A comparative analysis of PCR-based detection methods for avian malaria. J Parasitol 88:819–822
Ricklefs RE, Fallon SM (2002) Diversification and host switching in avian malaria parasites. Proc R Soc Lond B 269:885–892
Rintamäki PT, Ojanen O, Pakkala H et al (1998) Blood parasites of migrating Willow Warblers (Phylloscopus trochilus) at a stopover site. Can J Zool 76:984–988
Robbins CS, Sauer JR, Greenberg RS et al (1989) Population declines in North American birds that migrate to the neotropics. Proc Natl Acad Sci USA 86:7658–7662
Robinson S, Thompson F III, Donovan T et al (1995) Regional forest fragmentation and the nesting success of migratory birds. Science 267:1987–1990
Rubenstein DR, Chamberlain CP, Holmes RT et al (2002) Linking breeding and wintering ranges of a migratory songbird using stable isotopes. Science 295:1062–1065
Sillett TS, Holmes RT (2002) Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol 71:296–308
Slatkin M (2004) Gene flow and the geographic structure of natural populations. Science 236:787–792
Sol D, Jovani R, Torres J (2000) Geographical variation in blood parasites in feral pigeons: the role of vectors. Ecography 23:307–314
Super P, van Riper C III (1995) A comparison of avian hematozoan epizootiology in two California coastal scrub communities. J Wildl Dis 31:447–461
Swofford DL (1999) paup*: Phylogenetic analysis using parsimony (*and other methods). Sinauer, Sunderland, MA
Szymanski MM, Lovette IJ (2005) High lineage diversity and host sharing of malarial parasites in a local avian assemblage. J Parasitol 91:768–774
Tankersley R, Orvis K (2003) Modeling the geography of migratory pathways and stopover habitats for neotropical migratory birds. Conserv Ecol 7(1):7
Valkiunas G (2004) Avian malarial parasites and other haemosporidians. CRC Press
Waldenström J, Bensch S, Kiboi S et al (2002) Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Mol Ecol 11:1545–1554
Waldenström J, Bensch S, Hasselquist D et al (2004) A new nested polymerase chain reaction method very efficient in detecting plasmodium and haemoproteus infections from avian blood. J Parasitol 90:191–194
Wassenaar L, Hobson K (2000) Stable-carbon and hydrogen isotope ratios reveal breeding origins of red-winged blackbirds. Ecol Appl 10:911–916
Weatherhead P, Bennet G (1992) Ecology of parasitism of brown-headed cowbirds by haematozoa. Can J Zool 70:1–7
Webster MS, Marra PP, Haig SM et al (2002) Links between worlds: unraveling migratory connectivity. Trends Ecol Evol 17:76–83
Wennerberg L, Klaassen M, Lindstrom A (2002) Geographical variation and population structure in the white-rumped sandpiper Calidris fuscicollis as shown by morphology, mitochondrial DNA and carbon isotope ratios. Oecoloiga 131:390–390
Wiersch SC, Maier WA, Kampen H (2005) Plasmodium (Haemamoeba) cathemerium gene sequences for phylogenetic analysis of malaria parasites. Parasitol Res 95:90–94
Wirth T, Meyer A, Achtman M (2005) Deciphering host migrations and origins by means of their microbes. Mol Ecol 14:3289–3306
Woodworth B, Atkinson CT, LaPointe D et al (2005) Host population persistence in the face of introduced vector-borne diseases: Hawaii amakihi and avian malaria. Proc Natl Acad Sci USA 102:1531–1536
Acknowledgments
We would like to thank committee members, Catherine Schaeff and Kiho Kim, for intellectual insight and guidance. We would also like to thank two anonymous reviewers for their helpful comments. A small number of additional samples were contributed by Brian Olsen, Irby Lovette, and Rebecca Holberton. We would like to thank the various organizations that allowed us to sample common yellowthroats on their land: Aroostook National Wildlife Refuge, Iroquois National Wildlife Refuge, Paul Smith College of the Adirondacks, and the Pfeiffer Nature Center. We especially thank Daryl and Leslie Boness, who not only allowed us to sample on their beautiful property but also fed us well. We thank Carl McIntosh, Danielle Palmer, Farah Ishtiaq and Jon Beadell for help with various lab techniques. Funding was provided by the NIH (1R01GM063258) and an American University Mellon Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
The U.S. Government’s right to retain a non-exclusive, royalty-free license in and to any copyright is acknowledged.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pagenkopp, K.M., Klicka, J., Durrant, K.L. et al. Geographic variation in malarial parasite lineages in the common yellowthroat (Geothlypis trichas). Conserv Genet 9, 1577–1588 (2008). https://doi.org/10.1007/s10592-007-9497-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-007-9497-6