Abstract
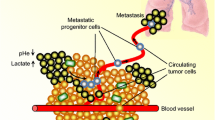
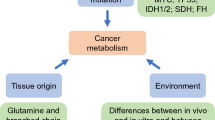
Metabolic reprogramming is a hallmark of cancer metastasis in which cancer cells manipulate their metabolic profile to meet the dynamic energetic requirements of the tumor microenvironment. Though cancer cell proliferation and migration through the extracellular matrix are key steps of cancer progression, they are not necessarily fueled by the same metabolites and energy production pathways. The two main metabolic pathways cancer cells use to derive energy from glucose, glycolysis and oxidative phosphorylation, are preferentially and plastically utilized by cancer cells depending on both their intrinsic metabolic properties and their surrounding environment. Mechanical factors in the microenvironment, such as collagen density, pore size, and alignment, and biochemical factors, such as oxygen and glucose availability, have been shown to influence both cell migration and glucose metabolism. As cancer cells have been identified as preferentially utilizing glycolysis or oxidative phosphorylation based on heterogeneous intrinsic or extrinsic factors, the relationship between cancer cell metabolism and metastatic potential is of recent interest. Here, we review current in vitro and in vivo findings in the context of cancer cell metabolism during migration and metastasis and extrapolate potential clinical applications of this work that could aid in diagnosing and tracking cancer progression in vivo by monitoring metabolism. We also review current progress in the development of a variety of metabolically targeted anti-metastatic drugs, both in clinical trials and approved for distribution, and highlight potential routes for incorporating our recent understanding of metabolic plasticity into therapeutic directions. By further understanding cancer cell energy production pathways and metabolic plasticity, more effective and successful clinical imaging and therapeutics can be developed to diagnose, target, and inhibit metastasis.




Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ATP:
-
Adenosine tri-phosphate
- Cav1:
-
Caveolin-1
- TCA:
-
Citric acid cycle
- CAT:
-
Collective to amoeboid transition
- EMT:
-
Epithelial to mesenchymal transition
- ECM:
-
Extracellular matrix
- FLIM:
-
Fluorescence lifetime imaging
- GLUT1:
-
Glucose transporter 1
- HIF-1:
-
Hypoxia-inducible factor-1
- IDH-2:
-
Isocitrate dehydrogenase 2
- LDH-A:
-
Lactate dehydrogenase A
- mTOR:
-
Mammalian target of rapamycin
- MMP:
-
Matrix metalloproteinase
- MAT:
-
Mesenchymal to amoeboid transition
- MCT4:
-
Monocarboxylate transporter 4
- NAD:
-
Nicotinamide adenine dinucleotide
- NADH:
-
Nicotinamide adenine dinucleotide+hydrogen
- OxPhos:
-
Oxidative phosphorylation
- PCG-1α:
-
Peroxisome proliferator-associated receptor gamma, coactivator 1-alpha
- PET:
-
Positron emission tomography
- PDH1:
-
Pyruvate dehydrogenase 1
- PDK1:
-
Pyruvate hydrogenase kinase 1
- ROS:
-
Reactive oxygen species
- TME:
-
Tumor microenvironment
- TNBC:
-
Triple negative breast cancer
- 18-FDG:
-
18-Fluorodeoxyglucose
References
Hapach LA, Mosier JA, Wang W, Reinhart-King CA (2019) Engineered models to parse apart the metastatic cascade. NPJ Precis Oncol 3:1–8
Zanotelli MR et al (2019) Energetic costs regulated by cell mechanics and confinement are predictive of migration path during decision-making. Nat Commun 10:1–12
Zanotelli MR et al (2018) Regulation of ATP utilization during metastatic cell migration by collagen architecture. MBoC 29:1–9
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Ann Rev Cell Dev Biol 27:441–464
Icard P et al (2018) How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat 38:1–11
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41:211–218
Sousa B, Pereira J, Paredes J (2019) The crosstalk between cell adhesion and cancer metabolism. Int J Mol Sci 20:1933
Lee SH, Dominguez R (2010) Regulation of actin cytoskeleton dynamics in cells. Mol Cells 29:311–325
Suzuki R, Hotta K, Oka K (2015) Spatiotemporal quantification of subcellular ATP levels in a single HeLa cell during changes in morphology. Sci Rep. https://doi.org/10.1038/srep16874
Passam F et al (2018) Mechano-redox control of integrin de-adhesion. Elife. https://doi.org/10.7554/eLife.34843
Fedotov S, Iomin A (2007) Migration and proliferation dichotomy in tumor-cell invasion. Phys Rev Lett 98:118101
Giese A et al (1996) Dichotomy of astrocytoma migration and proliferation. Int J Cancer 67:275–282
Zheng P-P, Severijnen L-A, van der Weiden M, Willemsen R, Kros JM (2009) Cell proliferation and migration are mutually exclusive cellular phenomena in vivo: implications for cancer therapeutic strategies. Cell Cycle 8:950–951
Garay T et al (2013) Cell migration or cytokinesis and proliferation?—Revisiting the “go or grow” hypothesis in cancer cells in vitro. Exp Cell Res 319:3094–3103
Lewis CA et al (2014) Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell 55:253–263
Webster KA (2003) Evolution of the coordinate regulation of glycolytic enzyme genes by hypoxia. J Exp Biol 206:2911–2922
Hay N (2016) Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer 16:635–649
Ge T et al (2020) The role of the pentose phosphate pathway in diabetes and cancer. Front. Endocrinol 11:365
Cluntun AA, Lukey MJ, Cerione RA, Locasale JW (2017) Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3:169–180
Paudel BB, Quaranta V (2019) Metabolic plasticity meets gene regulation. Proc Natl Acad Sci U S A 116:3370–3372
Koczula KM et al (2016) Metabolic plasticity in CLL: adaptation to the hypoxic niche. Leukemia 30:65–73
Jia D et al (2019) Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci USA 116:3909–3918
Warburg O (1925) The metabolism of carcinoma cells. J Cancer Res 9:148–163
Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21:297–308
Vaupel P, Schmidberger H, Mayer A (2019) The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol 95:912–919
Elstrom RL et al (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64:3892–3899
Shim H et al (1997) c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A 94:6658–6663
van Zijl F, Krupitza G, Mikulits W (2011) Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res Rev Mutat Res 728:23–34
Giampieri S et al (2009) Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11:1287–1296
te Boekhorst V et al (2020) Calpain-2 regulates hypoxia/HIF-induced amoeboid reprogramming and metastasis. bioRxiv. https://doi.org/10.1101/2020.01.06.892497
Chung Y-C et al (2016) Rab11 collaborates E-cadherin to promote collective cell migration and indicates a poor prognosis in colorectal carcinoma. Eur J Clin Invest 46:1002–1011
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374
Emad A et al (2020) Superior breast cancer metastasis risk stratification using an epithelial-mesenchymal-amoeboid transition gene signature. Breast Cancer Res 22:74
Tolde O et al (2018) Quantitative phase imaging unravels new insight into dynamics of mesenchymal and amoeboid cancer cell invasion. Sci Rep 8:12020
Parri M, Taddei ML, Bianchini F, Calorini L, Chiarugi P (2009) EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res 69:2072–2081
Haga H, Irahara C, Kobayashi R, Nakagaki T, Kawabata K (2005) Collective movement of epithelial cells on a collagen gel substrate. Biophys J 88:2250–2256
De Donatis A, Ranaldi F, Cirri P (2010) Reciprocal control of cell proliferation and migration. Cell Commun Signal 8:20
Hecht I et al (2015) Tumor invasion optimization by mesenchymal-amoeboid heterogeneity. Sci Rep 5:10622
Zhang J et al (2019) Energetic regulation of coordinated leader–follower dynamics during collective invasion of breast cancer cells. PNAS 116:7867–7872
LeBleu VS et al (2014) PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16:992–1003
Commander R et al (2020) Subpopulation targeting of pyruvate dehydrogenase and GLUT1 decouples metabolic heterogeneity during collective cancer cell invasion. Nat Commun 11:1–17
Carmeliet P, De Smet F, Loges S, Mazzone M (2009) Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol 6:315–326
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147:992–1009
Polacheck WJ, Zervantonakis IK, Kamm RD (2013) Tumor cell migration in complex microenvironments. Cell Mol Life Sci 70:1335–1356
Lintz M, Muñoz A, Reinhart-King CA (2017) The mechanics of single cell and collective migration of tumor cells. J Biomech Eng 139:0210051–0210059
Friedl P, Wolf K (2010) Plasticity of cell migration: a multiscale tuning model. J Cell Biol 188:11–19
Aiello NM et al (2018) EMT subtype influences epithelial plasticity and mode of cell migration. Dev Cell 45:681-695.e4
Wang Y, Zhou BP (2013) Epithelial-mesenchymal transition–-A hallmark of breast cancer metastasis. Cancer Hallm 1:38–49
Vincent-Salomon A, Thiery JP (2003) Host microenvironment in breast cancer development: epithelial–mesenchymal transition in breast cancer development. Breast Cancer Res 5:101–106
Shiraishi T et al (2015) Glycolysis is the primary bioenergetic pathway for cell motility and cytoskeletal remodeling in human prostate and breast cancer cells. Oncotarget 6:130–143
Berx G, Raspé E, Christofori G, Thiery JP, Sleeman JP (2007) Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin Exp Metastasis 24:587–597
Kim NH et al (2017) Snail reprograms glucose metabolism by repressing phosphofructokinase PFKP allowing cancer cell survival under metabolic stress. Nat Commun 8:14374
Cooke VG et al (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21:66–81
Yang M-H et al (2008) Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol 10:295–305
Mosier JA et al (2019) Extent of cell confinement in microtracks affects speed and results in differential matrix strains. Biophys J 117:1692–1701
Lee IJ et al (2006) Hepatocellular carcinoma model cell lines with two distinct migration modes. Biochem Biophys Res Commun 346:1217–1227
Huang B et al (2014) The three-way switch operation of Rac1/RhoA GTPase-based circuit controlling amoeboid-hybrid-mesenchymal transition. Sci Rep 4:6449
Holle AW et al (2019) Cancer cells invade confined microchannels via a self-directed mesenchymal-to-amoeboid transition. Nano Lett 19:2280–2290
Čermák V et al (2020) High-throughput transcriptomic and proteomic profiling of mesenchymal-amoeboid transition in 3D collagen. Sci Data 7:160
Thejer BM et al (2020) PGRMC1 phosphorylation affects cell shape, motility, glycolysis, mitochondrial form and function, and tumor growth. BMC Mol Cell Biol 21:24
Anesti V, Scorrano L (2006) The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta 1757:692–699
Bartolák-Suki E, Imsirovic J, Nishibori Y, Krishnan R, Suki B (2017) Regulation of mitochondrial structure and dynamics by the cytoskeleton and mechanical factors. Int J Mol Sci 18:1812
Ali MH, Pearlstein DP, Mathieu CE, Schumacker PT (2004) Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287:486–496
Kondo H et al (2021) Single-cell resolved imaging reveals intra-tumor heterogeneity in glycolysis, transitions between metabolic states, and their regulatory mechanisms. Cell Rep 34:108750
Kelley LC et al (2019) Adaptive F-actin polymerization and localized ATP production drive basement membrane invasion in the absence of MMPs. Dev Cell 48:313-328.e8
Cunniff B, McKenzie AJ, Heintz NH, Howe AK (2016) AMPK activity regulates trafficking of mitochondria to the leading edge during cell migration and matrix invasion. MBoC 27:2662–2674
Porporato PE et al (2014) A mitochondrial switch promotes tumor metastasis. Cell Rep 8:754–766
Yoshida S et al (2013) Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. PNAS 110:E1604–E1612
Krakhmal NV, Zavyalova MV, Denisov EV, Vtorushin SV, Perelmuter VM (2015) Cancer invasion: patterns and mechanisms. Acta Nat 7:17–28
Duda DG et al (2010) Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1016234107
Klameth L et al (2017) Small cell lung cancer: model of circulating tumor cell tumorospheres in chemoresistance. Sci Rep 7:5337
Plou J et al (2018) From individual to collective 3D cancer dissemination: roles of collagen concentration and TGF-β. Sci Rep 8:12723
Aceto N et al (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158:1110–1122
Wu J-S et al (2019) Cathepsin B defines leader cells during the collective invasion of salivary adenoid cystic carcinoma. Int J Oncol 54:1233–1244
Wolf K et al (2007) Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol 9:893–904
Glentis A et al (2017) Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat Commun 8:924
Shih W, Yamada S (2012) N-cadherin as a key regulator of collective cell migration in a 3D environment. Cell Adhes Migr 6:513–517
Elisha Y, Kalchenko V, Kuznetsov Y, Geiger B (2018) Dual role of E-cadherin in the regulation of invasive collective migration of mammary carcinoma cells. Sci Rep 8:4986
De Bock K et al (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154:651–663
Cruys B et al (2016) Glycolytic regulation of cell rearrangement in angiogenesis. Nat Commun 7:12240
Xie J et al (2014) Beyond Warburg effect—dual metabolic nature of cancer cells. Sci Rep 4:4927
Lu W et al (2012) Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol 10:e1001326
Payen VL, Porporato PE, Baselet B, Sonveaux P (2016) Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci 73:1333–1348
Busco G et al (2010) NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J 24:3903–3915
Schwager SC, Taufalele PV, Reinhart-King CA (2019) Cell-cell mechanical communication in cancer. Cel Mol Bioeng 12:1–14
Bear JE, Haugh JM (2014) Directed migration of mesenchymal cells: where signaling and the cytoskeleton meet. Curr Opin Cell Biol 0:74–82
Walker C, Mojares E, del Río Hernández A (2018) Role of extracellular matrix in development and cancer progression. Int J Mol Sci 19:3028
Grassian AR, Coloff JL, Brugge JS (2011) Extracellular matrix regulation of metabolism and implications for tumorigenesis. Cold Spring Harb Symp Quant Biol 76:313–324
Mah EJ, Lefebvre AEYT, McGahey GE, Yee AF, Digman MA (2018) Collagen density modulates triple-negative breast cancer cell metabolism through adhesion-mediated contractility. Sci Rep 8:17094
Weber GF (2016) Time and circumstances: cancer cell metabolism at various stages of disease progression. Front Oncol. https://doi.org/10.3389/fonc.2016.00257
Morris BA et al (2016) Collagen matrix density drives the metabolic shift in breast cancer cells. EBioMedicine 13:146–156
Haeger A, Krause M, Wolf K, Friedl P (2014) Cell jamming: collective invasion of mesenchymal tumor cells imposed by tissue confinement. Biochim Biophys Acta 1840:2386–2395
Wolf K et al (2009) Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 20:931–941
Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P (2008) Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol 130:1147–1154
Weigelin B, Bakker G-J, Friedl P (2012) Intravital third harmonic generation microscopy of collective melanoma cell invasion: principles of interface guidance and microvesicle dynamics. Intravital 1:32–43
Semenza GL, Roth PH, Fang HM, Wang GL (1994) Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 269:23757–23763
O’Rourke JF, Pugh CW, Bartlett SM, Ratcliffe PJ (1996) Identification of hypoxically inducible mRNAs in HeLa cells using differential-display PCR. Role of hypoxia-inducible factor-1. Eur J Biochem. 241:403–410
Lehmann S et al (2017) Hypoxia induces a HIF-1-dependent transition from collective-to-amoeboid dissemination in epithelial cancer cells. Curr Biol 27:392–400
Molavian HR, Kohandel M, Sivaloganathan S (2016) High concentrations of H2O2 make aerobic glycolysis energetically more favorable for cellular respiration. Front Physiol 7:362
Druzhkova IN et al (2016) The metabolic interaction of cancer cells and fibroblasts - coupling between NAD(P)H and FAD, intracellular pH and hydrogen peroxide. Cell Cycle 15:1257–1266
Mailloux RJ et al (2007) The tricarboxylic acid cycle, an ancient metabolic network with a novel twist. PLoS ONE 2:e690
Takatani-Nakase T, Matsui C, Maeda S, Kawahara S, Takahashi K (2014) High glucose level promotes migration behavior of breast cancer cells through zinc and its transporters. PLoS ONE 9:e90136
Lyssiotis CA, Kimmelman AC (2017) Metabolic interactions in the tumor microenvironment. Trends Cell Biol 27:863–875
Ho P-C et al (2015) Phosphoenolpyruvate Is a metabolic checkpoint of anti-tumor T cell responses. Cell 162:1217–1228
Chang C-H et al (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162:1229–1241
Gould CM, Courtneidge SA (2014) Regulation of invadopodia by the tumor microenvironment. Cell Adh Migr 8:226–235
Debreova M et al (2019) CAIX regulates invadopodia formation through both a pH-dependent mechanism and interplay with actin regulatory proteins. Int J Mol Sci 20:2745
Nelson CM et al (2005) Emergent patterns of growth controlled by multicellular form and mechanics. PNAS 102:11594–11599
Kato Y et al (2007) Acidic extracellular pH increases calcium influx-triggered phospholipase D activity along with acidic sphingomyelinase activation to induce matrix metalloproteinase-9 expression in mouse metastatic melanoma. FEBS J 274:3171–3183
de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J (2019) Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. https://doi.org/10.3389/fonc.2019.01143
Wu H, Ying M, Hu X (2016) Lactic acidosis switches cancer cells from aerobic glycolysis back to dominant oxidative phosphorylation. Oncotarget 7:40621–40629
Khacho M et al (2014) Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat Commun 5:3550
Sotgia F et al (2012) Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol 7:423–467
Mougeolle A et al (2015) Oxidative stress induces caveolin 1 degradation and impairs caveolae functions in skeletal muscle cells. PLoS ONE 10:e0122654
Pavlides S et al (2009) The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8:3984–4001
Martinez-Outschoorn UE et al (2011) Anti-estrogen resistance in breast cancer is induced by the tumor microenvironment and can be overcome by inhibiting mitochondrial function in epithelial cancer cells. Cancer Biol Ther 12:924–938
Jiang E et al (2019) Tumoral microvesicle–activated glycometabolic reprogramming in fibroblasts promotes the progression of oral squamous cell carcinoma. FASEB J 33:5690–5703
Schwager SC et al (2019) Matrix stiffness regulates microvesicle-induced fibroblast activation. Am J Physiol Cell Physiol 317:C82–C92
Sedgwick AE, Clancy JW, Olivia Balmert M, D’Souza-Schorey C (2015) Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Sci Rep 5:14748
Begum HM et al (2019) Spatial regulation of mitochondrial heterogeneity by stromal confinement in micropatterned tumor models. Sci Rep 9:11187
Erdogan B et al (2017) Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol 216:3799–3816
Otranto M et al (2012) The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr 6:203–219
Provenzano PP et al (2006) Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 4:38
Menk AV et al (2018) Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep 22:1509–1521
Bantug GR, Galluzzi L, Kroemer G, Hess C (2018) The spectrum of T cell metabolism in health and disease. Nat Rev Immunol 18:19–34
Lim AR, Rathmell WK, Rathmell JC (2020) The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. Elife 9:e55185
Macintyre AN et al (2014) The glucose transporter glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab 20:61–72
Angiari S et al (2019) Regulation of T cell activation and pathogenicity by dimeric pyruvate kinase M2 (PKM2). J Immunol 202:125.11
Cavalli LR, Varella-Garcia M, Liang BC (1997) Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell Growth Differ 8:1189–1198
Morais R et al (1994) Tumor-forming ability in athymic nude mice of human cell lines devoid of mitochondrial DNA. Cancer Res 54:3889–3896
Chen EI et al (2007) Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res 67:1472–1486
Porporato PE, Sonveaux P (2015) Paving the way for therapeutic prevention of tumor metastasis with agents targeting mitochondrial superoxide. Mol Cell Oncol 2:e968043
Li AM et al (2020) Metabolic profiling reveals a dependency of human metastatic breast cancer on mitochondrial serine and one-carbon unit metabolism. Mol Cancer Res 18:599–611
Rademaker G et al (2019) Myoferlin contributes to the metastatic phenotype of pancreatic cancer cells by enhancing their migratory capacity through the control of oxidative phosphorylation. Cancers 11:853
Zhang T et al (2018) A small molecule targeting myoferlin exerts promising anti-tumor effects on breast cancer. Nat Commun 9:3726
Davis RT et al (2020) Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat Cell Biol 22:310–320
Huang M, Xiong H, Luo D, Xu B, Liu H (2020) CSN5 upregulates glycolysis to promote hepatocellular carcinoma metastasis via stabilizing the HK2 protein. Exp Cell Res 388:111876
Wiel C et al (2019) BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 178:330–345
Dupuy F et al (2015) PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab 22:577–589
Kim HM, Jung WH, Koo JS (2014) Site-specific metabolic phenotypes in metastatic breast cancer. J Transl Med 12:354
Stein EM et al (2017) Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130:722–731
Heredia V et al (2017) AG-120, a novel IDH1 targeted molecule, inhibits invasion and migration of chondrosarcoma cells in vitro. Ann Oncol 28:v538
Beloueche-Babari M et al (2020) Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br J Cancer 122:895–903
Kong SC et al (2016) Monocarboxylate transporters MCT1 and MCT4 regulate migration and invasion of pancreatic ductal adenocarcinoma cells. Pancreas 45:1036–1047
Gao L et al (2020) CPI-613 rewires lipid metabolism to enhance pancreatic cancer apoptosis via the AMPK-ACC signaling. J Exp Clin Cancer Res. https://doi.org/10.1186/s13046-020-01579-x
Saraei P, Asadi I, Kakar MA, Moradi-Kor N (2019) The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manag Res 11:3295–3313
Alimova IN et al (2009) Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 8:909–915
Jang SY et al (2014) Metformin inhibits tumor cell migration via down-regulation of MMP9 in tamoxifen-resistant breast cancer cells. Anticancer Res 34:4127–4134
Raninga PV et al (2020) Marizomib suppresses triple-negative breast cancer via proteasome and oxidative phosphorylation inhibition. Theranostics 10:5259–5275
Huang Q et al (2020) Novel ginsenoside derivative 20( S )-Rh2E2 suppresses tumor growth and metastasis in vivo and in vitro via intervention of cancer cell energy metabolism. Cell Death Dis 11:1–19
Langer A (2010) A systematic review of PET and PET/CT in oncology: a way to personalize cancer treatment in a cost-effective manner? BMC Health Serv Res 10:283
Zhu A, Lee D, Shim H (2011) Metabolic PET imaging in cancer detection and therapy response. Semin Oncol 38:55–69
Chen K, Chen X (2011) Positron emission tomography imaging of cancer biology: current status and future prospects. Semin Oncol 38:70–86
Moses WW (2011) Fundamental limits of spatial resolution in PET. Nucl Instrum Methods Phys Res A 648(Supplement 1):S236–S240
Culverwell AD, Scarsbrook AF, Chowdhury FU (2011) False-positive uptake on 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) positron-emission tomography/computed tomography (PET/CT) in oncological imaging. Clin Radiol 66:366–382
Jose C, Bellance N, Rossignol R (2011) Choosing between glycolysis and oxidative phosphorylation: a tumor’s dilemma? Biochim Biophys Acta 1807:552–561
Feng H et al (2019) Nuclear imaging of glucose metabolism: beyond 18F-FDG. Contrast Media Mol Imaging 2019:1–12
Croteau E et al (2016) PET metabolic biomarkers for cancer. Biomark Cancer 8:61–69
Spick C, Herrmann K, Czernin J (2016) Evaluation of prostate cancer with 11C-acetate PET/CT. J Nucl Med 57:30S-37S
Karaayvaz M et al (2018) Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat Commun 9:3588
Cheng J et al (2020) TRIM21 and PHLDA3 negatively regulate the crosstalk between the PI3K/AKT pathway and PPP metabolism. Nat Commun 11:1880
Gerber T et al (2017) Mapping heterogeneity in patient-derived melanoma cultures by single-cell RNA-seq. Oncotarget 8:846–862
El-Mir MY et al (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 275:223–228
Mediani L et al (2016) Reversal of the glycolytic phenotype of primary effusion lymphoma cells by combined targeting of cellular metabolism and PI3K/Akt/ mTOR signaling. Oncotarget 7:5521–5537
Raez LE et al (2013) A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 71:523–530
Chapiro J et al (2014) Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer. Clin Cancer Res 20:6406–6417
Baggstrom MQ et al (2011) A phase II study of AT-101 (gossypol) in chemotherapy-sensitive recurrent extensive stage small cell lung cancer (ES-SCLC). J Thorac Oncol 6:1757–1760
Lycan TW et al (2016) A phase II clinical trial of CPI-613 in patients with relapsed or refractory small cell lung carcinoma. PLoS ONE 11:e0164244
Velpula KK, Bhasin A, Asuthkar S, Tsung AJ (2013) Combined targeting of PDK1 and EGFR triggers regression of glioblastoma by reversing the Warburg effect. Cancer Res 73:7277–7289
Chu QS-C et al (2015) A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs 33:603–610
Moore Z et al (2015) NAMPT inhibition sensitizes pancreatic adenocarcinoma cells to tumor-selective, PAR-independent metabolic catastrophe and cell death induced by β-lapachone. Cell Death Dis 6:e1599
Golub D et al (2019) Mutant isocitrate dehydrogenase inhibitors as targeted cancer therapeutics. Front Oncol. https://doi.org/10.3389/fonc.2019.00417
DiNardo CD et al (2018) Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 378:2386–2398
Amadori D et al (1998) Modulating effect of lonidamine on response to doxorubicin in metastatic breast cancer patients: results from a multicenter prospective randomized trial. Breast Cancer Res Treat 49:209–217
Nath K et al (2015) Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed 28:281–290
Spencer A et al (2018) A phase 1 clinical trial evaluating marizomib, pomalidomide and low-dose dexamethasone in relapsed and refractory multiple myeloma (NPI-0052-107): final study results. Br J Haematol 180:41–51
Gee JR et al (2017) A phase II randomized, double-blind, presurgical trial of polyphenon E in bladder cancer patients to evaluate pharmacodynamics and bladder tissue biomarkers. Cancer Prev Res 10:298–307
Berman AY, Motechin RA, Wiesenfeld MY, Holz MK (2017) The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol. https://doi.org/10.1038/s41698-017-0038-6
Sato A, Asano T, Ito K, Asano T (2012) Ritonavir interacts with bortezomib to enhance protein ubiquitination and histone acetylation synergistically in renal cancer cells. Urology 79(966):e13-21
Vander Heiden MG et al (2010) Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol 79:1118–1124
Clem B et al (2008) Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 7:110–120
Clem BF et al (2013) Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther 12:1461–1470
Redman RA, Pohlmann PR, Kurman MR, Tapolsky G, Chesney JA (2015) A phase I, dose-escalation, multi-center study of PFK-158 in patients with advanced solid malignancies explores a first-in-man inhbibitor of glycolysis. JCO 33:TPS2606
Ocaña MC, Martínez-Poveda B, Marí-Beffa M, Quesada AR, Medina MÁ (2020) Fasentin diminishes endothelial cell proliferation, differentiation and invasion in a glucose metabolism-independent manner. Sci Rep 10:6132
Wang Y et al (2016) GEN-27, a newly synthetic isoflavonoid, inhibits the proliferation of colon cancer cells in inflammation microenvironment by suppressing NF-κB pathway. Mediat Inflamm 2016:2853040
Kumagai S, Narasaki R, Hasumi K (2008) Glucose-dependent active ATP depletion by koningic acid kills high-glycolytic cells. Biochem Biophys Res Commun 365:362–368
Ozerlat I (2011) Targeted therapy of glucose uptake via GLUT1 kills RCC cells. Nat Rev Urol 8:471–471
Liu Y et al (2012) A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther 11:1672–1682
National Library of Medicine (U.S.) (2019) A study of CPI-613 for patients with relapsed or refractory burkitt lymphoma/leukemia or high-grade B-cell lymphoma with high-risk translocations. https://clinicaltrials.gov/ct2/show/NCT03793140. Accessed 17 Nov 2020
Acknowledgements
This work was supported by funding from the NIH (GM131178) to C.A.R.-K., the W.M. Keck Foundation to C.A.R.-K., and by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1937963 awarded to J.A.M. and S.C.S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mosier, J.A., Schwager, S.C., Boyajian, D.A. et al. Cancer cell metabolic plasticity in migration and metastasis. Clin Exp Metastasis 38, 343–359 (2021). https://doi.org/10.1007/s10585-021-10102-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-021-10102-1