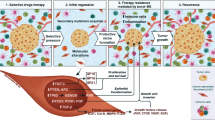
Abstract
Cancer heterogeneity is a result of genetic mutations within the cancer cells. Their proliferation is not only driven by autocrine functions but also under the influence of cancer microenvironment, which consists of normal stromal cells such as infiltrating immune cells, cancer-associated fibroblasts, endothelial cells, pericytes, vascular and lymphatic channels. The relationship between cancer cells and cancer microenvironment is a critical one and we are just on the verge to understand it on a molecular level. Cancer microenvironment may serve as a selective force to modulate cancer cells to allow them to evolve into more aggressive clones with ability to invade the lymphatic or vascular channels to spread to regional lymph nodes and distant sites. It is important to understand these steps of cancer evolution within the cancer microenvironment towards invasion so that therapeutic strategies can be developed to control or stop these processes.




Similar content being viewed by others
Abbreviations
- MBM:
-
Brain metastasis
- ANGPTL4:
-
Angiopoietin-like 4
- CysC:
-
Cystatin C
- CLDN1:
-
Claudin-1
- DTCs:
-
Disseminated tumor cells
- ECM:
-
Extracellular matrix
- MM:
-
Malignant melanoma
- bHLH:
-
Helix-loop-helix
- CPI:
-
Checkpoint inhibitors
- IO:
-
Immunoncology
- TAMPP:
-
T-cell activation marker proficiency panel
- ROI:
-
Regions of interest
- CNN:
-
Convolutional neural networks
- BC:
-
Breast cancer
- AA:
-
African American
- EA:
-
White or European American
- TNBC:
-
Triple-negative BC
- ICSBCS:
-
International Center for the Study of Breast Cancer Subtypes
- TST:
-
Trans-Atlantic Slave Trade
- DARC/ACKR1 :
-
Duffy Antigen Receptor for Chemokines/Atypical Chemokine Receptor 1 gene
- IMC:
-
Imaging mass cytometry
- RNA-Seq:
-
RNA sequencing
- GTEx:
-
Genotype-Tissue Expression database
- Treehouse CARE:
-
Treehouse Comparative Analysis of RNA Expression
- CRISPR/Cas9:
-
Clustered Regularly-Interspaced Short Palindromic Repeats
References
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322
Darwin C (1859) On the origin of species by means of natural selection, or preservation of favoured races in the struggle for life. John Murray, London
Leong SP, Aktipis A, Maley C (2018) Cancer initiation and progression within the cancer microenvironment. Clin Exp Metastasis 35(5–6):361–367
Boire A et al (2020) Brain metastasis. Nat Rev Cancer 20(1):4–11
Orozco JIJ et al (2018) Epigenetic profiling for the molecular classification of metastatic brain tumors. Nat Commun 9(1):4627
Valiente M et al (2018) The evolving landscape of brain metastasis. Trends Cancer 4(3):176–196
Winkler F (2015) The brain metastatic niche. J Mol Med (Berl) 93(11):1213–1220
Izraely S, Klein A, Sagi-Assif O, Meshel T, Tsarfaty G, Hoon DSB, Witz IP (2010) The metastatic microenvironment: brain-residing melanoma metastasis and dormant micrometastasis. Int J Cancer 130(1–2):107–114. https://doi.org/10.1016/j.imlet.2009.12.003
Neuditschko B et al (2020) The challenge of classifying metastatic cell properties by molecular profiling exemplified with cutaneous melanoma cells and their cerebral metastasis from patient derived mouse xenografts. Mol Cell Proteomics 19(3):478–489
Izraely S, Witz IP (2021) Site-specific metastasis: a cooperation between cancer cells and the metastatic microenvironment. Int J Cancer 148(6):1308–1322. https://doi.org/10.1002/ijc.33247
Izraely S et al (2017) ANGPTL4 promotes the progression of cutaneous melanoma to brain metastasis. Oncotarget 8(44):75778–75796
Tan MJ et al (2012) Emerging roles of angiopoietin-like 4 in human cancer. Mol Cancer Res 10(6):677–688
Zlotnik A, Burkhardt AM, Homey B (2011) Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol 11(9):597–606
Izraely S, Klein A, Sagi-Assif O, Meshel T, Tsarfaty G, Hoon DSB, Witz IP (2010) Chemokine–chemokine receptor axes in melanoma brain metastasis. Immunol Lett 130(1–2):107–114. https://doi.org/10.1016/j.imlet.2009.12.003
Klein A et al (2012) The metastatic microenvironment: brain-derived soluble factors alter the malignant phenotype of cutaneous and brain-metastasizing melanoma cells. Int J Cancer 131(11):2509–2518
Klein A et al (2017) CCR4 is a determinant of melanoma brain metastasis. Oncotarget 8(19):31079–31091
Moshe A et al (2018) Cystatin C takes part in melanoma-microglia cross-talk: possible implications for brain metastasis. Clin Exp Metastasis 35(5–6):369–378
Kopitz C et al (2005) Reduction of experimental human fibrosarcoma lung metastasis in mice by adenovirus-mediated cystatin C overexpression in the host. Cancer Res 65(19):8608–8612
Huh CG et al (1999) Decreased metastatic spread in mice homozygous for a null allele of the cystatin C protease inhibitor gene. Mol Pathol 52(6):332–340
Klein A et al (2015) Astrocytes facilitate melanoma brain metastasis via secretion of IL-23. J Pathol 236(1):116–127
Langowski JL et al (2006) IL-23 promotes tumour incidence and growth. Nature 442(7101):461–465
Moshe A, Izraely S, Sagi-Assif O, Malka S, Ben-Menachem S, Meshel T, Pasmanik-Chor M, Hoon DSB, Witz IP, (2020) Inter-tumor heterogeneity—melanomas respond differently to GM-CSF-mediated activation. Cells 9(7):1683. https://doi.org/10.3390/cells9071683
Hoeller C et al (2016) Systematic review of the use of granulocyte-macrophage colony-stimulating factor in patients with advanced melanoma. Cancer Immunol Immunother 65(9):1015–1034
Chang YC et al (2018) Roles of aldolase family genes in human cancers and diseases. Trends Endocrinol Metab 29(8):549–559
Ramos RI et al (2020) Upregulation of cell surface GD3 ganglioside phenotype is associated with human melanoma brain metastasis. Mol Oncol 14(8):1760–1778
Bhat AA et al (2020) Claudin-1, a double-edged sword in cancer. Int J Mol Sci 21(2):569
Izraely S et al (2015) The metastatic microenvironment: Claudin-1 suppresses the malignant phenotype of melanoma brain metastasis. Int J Cancer 136(6):1296–1307
Stebbing J, Filipovic A, Giamas G (2013) Claudin-1 as a promoter of EMT in hepatocellular carcinoma. Oncogene 32(41):4871–4872
Almad A, Maragakis NJ (2018) A stocked toolbox for understanding the role of astrocytes in disease. Nat Rev Neurol 14(6):351–362
Fidler IJ et al (2010) The brain microenvironment and cancer metastasis. Mol Cells 30(2):93–98
Eyo UB, Wu LJ (2019) Microglia: lifelong patrolling immune cells of the brain. Prog Neurobiol 179:101614
Izraely S et al (2019) The metastatic microenvironment: melanoma-microglia cross-talk promotes the malignant phenotype of melanoma cells. Int J Cancer 144(4):802–817
Ohab JJ et al (2006) A neurovascular niche for neurogenesis after stroke. J Neurosci 26(50):13007–13016
Prakash R et al (2019) Regeneration enhances metastasis: a novel role for neurovascular signaling in promoting melanoma brain metastasis. Front Neurosci 13:297
Sleeman JP et al (2012) Concepts of metastasis in flux: the stromal progression model. Semin Cancer Biol 22(3):174–186
Sleeman JP (2012) The metastatic niche and stromal progression. Cancer Metastasis Rev 31(3–4):429–440
Bethan Psaila DL (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9:285–292
Celia-Terrassa T, Kang Y (2018) Metastatic niche functions and therapeutic opportunities. Nat Cell Biol 20(8):868–877
Hoye AM, Erler JT (2016) Structural ECM components in the premetastatic and metastatic niche. Am J Physiol Cell Physiol 310(11):C955–C967
Levental KR et al (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139(5):891–906
Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, Yang J (2015) Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat Cell Biol 17(5):678–688. https://doi.org/10.1038/ncb3157
Zaman MH, Trapani LM, Sieminski AL, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P (2006) Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA 103(29):10889–10894. https://doi.org/10.1073/pnas.0604460103
Barkan D, Green JE, Chambers AF (2010) Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer 46(7):1181–1188
Malanchi I et al (2011) Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481(7379):85–89
Oskarsson T et al (2011) Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 17(7):867–874
Hoshino A et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527(7578):329–335
Savoia P et al (2009) Skin metastases of malignant melanoma: a clinical and prognostic survey. Melanoma Res 19(5):321–326
Sedlmeier G, Al-Rawi V, Buchert J, Yserentant K, Rothley M, Steshina A, Gräßle S, Wu RL, Hurrle T, Richer W, Decraene C, Thiele W, Utikal J, Abuillan W, Tanaka M, Herten DP, Hill CS, Garvalov BK, Jung N, Bräse S, Sleeman JP (2020) Id1 and Id3 are regulated through matrix-assisted autocrine BMP signaling and represent therapeutic targets in melanoma. Adv Ther 4(2):2000065. https://doi.org/10.1002/adtp.202000065
Hollnagel A et al (1999) Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 274(28):19838–19845
Liang YY, Brunicardi FC, Lin X (2009) Smad3 mediates immediate early induction of Id1 by TGF-beta. Cell Res 19(1):140–148
Sun X-H, Copeland NG, Jenkins NA, Baltimore D (1991) Id proteins Idl and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol 11(11):5603–5611. https://doi.org/10.1128/MCB.11.11.5603
Gupta GP, Perk J, Acharyya S, de Candia P, Mittal V, Todorova-Manova K, Brogi E, Gerald WL, Benezra R, Massague J (2007) ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci USA 104(49):19506–19511. https://doi.org/10.1073/pnas.0709185104
Lai X et al (2014) Inhibitor of DNA-binding protein 1 knockdown arrests the growth of colorectal cancer cells and suppresses hepatic metastasis in vivo. Oncol Rep 32(1):79–88
O’Brien CA et al (2012) ID1 and ID3 regulate the self-renewal capacity of human colon cancer-initiating cells through p21. Cancer Cell 21(6):777–792
Roschger C, Cabrele C (2017) The Id-protein family in developmental and cancer-associated pathways. Cell Commun Signal 15(1):7
Straume O, Akslen LA (2005) Strong expression of ID1 protein is associated with decreased survival, increased expression of ephrin-A1/EPHA2, and reduced thrombospondin-1 in malignant melanoma. Br J Cancer 93(8):933–938
Pan L, Sato S, Frederick JP, Sun X-H, Zhuang Y (1999) Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol 19(9):5969–5980. https://doi.org/10.1128/MCB.19.9.5969
Darvin P et al (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50(12):1–11
Wolchok JD et al (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377(14):1345–1356
Garon EB et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372(21):2018–2028
Wei SC et al (2017) Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 170(6):1120–1133 e17
Galon J et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Pagès F et al (2018) International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391(10135):2128–2139
Mlecnik B et al (2020) Multicenter International Society for immunotherapy of cancer study of the consensus immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol 38(31):3638–3651
Samstein RM et al (2019) Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 51(2):202–206
Li K et al (2020) Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int 20:16
Diggs LP, Hsueh EC (2017) Utility of PD-L1 immunohistochemistry assays for predicting PD-1/PD-L1 inhibitor response. Biomark Res 5:12
Taube JM, Akturk G, Angelo M, et al (2020) The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation. J Immunother Cancer 8:e000155. https://doi.org/10.1136/jitc-2019-000155
Hoyt C et al (2019) Abstract LB-318: Multi-institutional TSA-amplified Multiplexed Immunofluorescence Reproducibility Evaluation (MITRE study): reproducibility assessment of an automated multiplexed immunofluorescence slide staining, imaging, and analysis workflow. Cancer Res 79:LB-LB-318
Pirici D et al (2009) Antibody elution method for multiple immunohistochemistry on primary antibodies raised in the same species and of the same subtype. J Histochem Cytochem 57(6):567–575
Giesen C et al (2014) Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods 11(4):417–425
Toki MI et al (2017) Proof of the quantitative potential of immunofluorescence by mass spectrometry. Lab Investig 97(3):329–334
Goltsev Y et al (2018) Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174(4):968–981 e15
Feng Z, Seliger B, Bernard AF (2017) Multiparametric immune profiling in HPV-oral squamous cell cancer. JCI Insight 2(14):e93652. https://doi.org/10.1172/jci.insight.93652
Hum L et al (2021) Cumulative suppressive index as a predictor of relapse free survival and overall survival in Human Papilloma Virus-negative oral squamous cell carcinomas with negative resection margins. Head Neck 43(2):568–576
Perou CM et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Sorlie T et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874
Parker JS et al (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27(8):1160–1167
Newman LA (2016) Parsing the etiology of breast cancer disparities. J Clin Oncol 34(9):1013–1014
DeSantis CE et al (2019) Breast cancer statistics, 2019. CA Cancer J Clin 69(6):438–451
DeSantis CE et al (2016) Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin 66(1):31–42
Allott EH et al (2018) Frequency of breast cancer subtypes among African American women in the AMBER consortium. Breast Cancer Res 20(1):12
Ihemelandu CU et al (2007) Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival. J Surg Res 143(1):109–118
Costa RLB, Gradishar WJ (2017) Triple-negative breast cancer: current practice and future directions. J Oncol Pract 13(5):301–303
Torre LA et al (2017) Global cancer in women: burden and trends. Cancer Epidemiol Biomark Prev 26(4):444–457
Jemal A, Fedewa SA (2012) Is the prevalence of ER-negative breast cancer in the US higher among Africa-born than US-born black women? Breast Cancer Res Treat 135(3):867–873
Newman LA et al (2019) Hereditary susceptibility for triple negative breast cancer associated with Western Sub-Saharan African ancestry: results from an International Surgical Breast Cancer Collaborative. Ann Surg 270(3):484–492
Jiagge E et al (2016) Comparative analysis of breast cancer phenotypes in African American, White American, and West versus East African patients: correlation between African ancestry and triple-negative breast cancer. Ann Surg Oncol 23(12):3843–3849
Al-Alem U et al (2014) Association of genetic ancestry with breast cancer in ethnically diverse women from Chicago. PLoS One 9(11):e112916
Kosoy R et al (2009) Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat 30(1):69–78
Nassir R et al (2009) An ancestry informative marker set for determining continental origin: validation and extension using human genome diversity panels. BMC Genet 10:39
Mersha TB, Abebe T (2015) Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics 9:1
Newman LA, Carpten J (2018) Integrating the genetics of race and ethnicity into cancer research: trailing Jane and John Q. Public. JAMA Surg 153(4):299–300
Martin DN et al (2009) Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One 4(2):e4531
Kim G et al (2020) The contribution of race to breast tumor microenvironment composition and disease progression. Front Oncol 10:1022
Nedelec Y et al (2016) Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell 167(3):657–669 e21
Davis M et al (2018) AR negative triple negative or “quadruple negative” breast cancers in African American women have an enriched basal and immune signature. PLoS One 13(6):e0196909
Davis M, Martini R, Newman L, Elemento O, White J, Verma A, Datta I, Adrianto I, Chen Y, Gardner K, Kim HG, Colomb WD, Eltoum IE, Frost AR, Grizzle WE, Sboner A, Manne U, Yates C (2020) Identification of distinct heterogenic subtypes and molecular signatures associated with African ancestry in triple negative breast cancer using quantified genetic ancestry models in admixed race populations. Cancers (Basel) 12(5):1220. https://doi.org/10.3390/cancers12051220
Livingstone FB (1984) The Duffy blood groups, vivax malaria, and malaria selection in human populations: a review. Hum Biol 56(3):413–425
Tournamille C et al (1995) Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet 10(2):224–228
Howes RE et al (2011) The global distribution of the Duffy blood group. Nat Commun 2:266
Lu ZH et al (1995) The promiscuous chemokine binding profile of the Duffy antigen/receptor for chemokines is primarily localized to sequences in the amino-terminal domain. J Biol Chem 270(44):26239–26245
Nibbs RJ, Graham GJ (2013) Immune regulation by atypical chemokine receptors. Nat Rev Immunol 13(11):815–829
Peiper SC et al (1995) The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J Exp Med 181(4):1311–1317
Pruenster M et al (2009) The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol 10(1):101–108
Jenkins BD et al (2019) Atypical chemokine receptor 1 (DARC/ACKR1) in breast tumors is associated with survival, circulating chemokines, tumor-infiltrating immune cells, and African ancestry. Cancer Epidemiol Biomark Prev 28(4):690–700
Filbin M, Monje M (2019) Developmental origins and emerging therapeutic opportunities for childhood cancer. Nat Med 25(3):367–376
Grobner SN et al (2018) The landscape of genomic alterations across childhood cancers. Nature 555(7696):321–327
Vaske OM et al (2019) Comparative tumor RNA sequencing analysis for difficult-to-treat pediatric and young adult patients with cancer. JAMA Netw Open 2(10):e1913968
Newton Y, Rassekh SR, Deyell RJ, Shen Y, Jones MR, Dunham C, Yip S, Leelakumari S, Zhu J, McColl D, Swatloski T, Salama SR, Ng T, Hendson G, Lee AF, Ma Y, Moore R, Mungall AJ, Haussler D, Stuart JM, Jantzen C, Laskin J, Jones SJM, Marra MA, Morozova O (2018) Comparative RNA-sequencing analysis benefits a pediatric patient with relapsed cancer. JCO Precis Oncol 2:1–16
Consortium GT et al (2017) Genetic effects on gene expression across human tissues. Nature 550(7675):204–213
Ford AM et al (2009) The TEL-AML1 leukemia fusion gene dysregulates the TGF-beta pathway in early B lineage progenitor cells. J Clin Investig 119(4):826–836
Treehouse Public Data. https://treehousegenomics.soe.ucsc.edu/public-data/
Vivian J et al (2017) Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol 35(4):314–316
Liang L et al (2015) Gliomatosis peritonei: a clinicopathologic and immunohistochemical study of 21 cases. Mod Pathol 28(12):1613–1620
Nevelius E (2020) Press release: the Nobel Prize in Chemistry 2020, in Genetic scissors: a tool for rewriting the code of life. The Royal Swedish Academy of Sciences, Stockholm
Fellmann C (2019) Emerging roles of Crispr-cas9 in precision-oncology. In: 8th International Cancer Metastasis Congress. San Francisco
Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8(5):317–327
Jinek M et al (2012) A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–820
Channel TNPY (2020) Nobel Lecture: Emmanuelle Charpentier, Nobel Prize in Chemistry 2020. Youtube. https://www.youtube.com/watch?v=3POrtQEpV2s
Channel TNPY (2020) Nobel Lecture: Jennifer Doudna, Nobel Prize in Chemistry 2020. Youtube.com. https://www.youtube.com/watch?v=KSrSIErIxMQ
Makarova KS et al (2015) An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol 13(11):722–736
Jiang W, Marraffini LA (2015) CRISPR-Cas: new tools for genetic manipulations from bacterial immunity systems. Annu Rev Microbiol 69:209–228
Sternberg SH et al (2015) Conformational control of DNA target cleavage by CRISPR-Cas9. Nature 527(7576):110–113
Mali P et al (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826
Jinek M et al (2013) RNA-programmed genome editing in human cells. Elife 2:e00471
Cong Le et al (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–822
Cho SW et al (2013) Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol 31(3):230–232
Fellmann C et al (2017) Cornerstones of CRISPR-Cas in drug discovery and therapy. Nat Rev Drug Discov 16(2):89–100
McBride DA et al (2019) Applications of molecular engineering in T-cell-based immunotherapies. Wiley Interdiscip Rev Nanomed Nanobiotechnol 11(5):e1557
Triolo VA (1965) Nineteenth century foundations of cancer research advances in tumor pathology, nomenclature, and theories of oncogenesis. Cancer Res 25:75–106
Dellinger MT, Witte MH (2018) Lymphangiogenesis, lymphatic systemomics, and cancer: context, advances and unanswered questions. Clin Exp Metastasis 35(5–6):419–424
Acknowledgements
Isaac P. Witz, Orit Sagi-Assif and Sivan Izraely thank their colleagues Shlomit Ben-Menachem and Tsipi Meshel, and the Laboratory of Tumor Microenvironment & Metastasis Research for their valuable help and support.
Funding
The research conducted by the authors is supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (Needham, MA, USA).
Author information
Authors and Affiliations
Contributions
Equal contribution regarding data/information collection, analysis and writing among the listed authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable for this review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presented at the 8th International Cancer Metastasis Congress in San Francisco, CA, USA from October 25–27, 2019 (http://www.cancermetastasis.org). To be published in an upcoming Special Issue of Clinical and Experimental Metastasis: Novel Frontiers in Cancer Metastasis.
Rights and permissions
About this article
Cite this article
Leong, S.P., Witz, I.P., Sagi-Assif, O. et al. Cancer microenvironment and genomics: evolution in process. Clin Exp Metastasis 39, 85–99 (2022). https://doi.org/10.1007/s10585-021-10097-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-021-10097-9