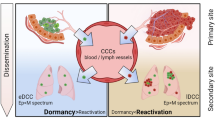
Abstract
Patient-derived orthotopic xenograft (PDOX) models have been verified as a useful method for studying human cancers in mice. Previous studies on the extent of metastases in these models have been limited by the necessity of welfare euthanasia (primary tumors reaching threshold size), at which point metastases may only be micrometers in diameter, few in number, and solely identified by step-sectioning of formalin-fixed paraffin-embedded tissue. These small micro-metastases are less suitable for many downstream molecular analyses than macro-metastases. Resection of the primary tumor by survival surgery has been proven to allow further time for metastases to grow. Although PDOX models of triple-negative breast cancer (TNBC) shed circulating tumor cells (CTCs) into the bloodstream and metastasize, similar to human TNBC, little data has been collected in these TNBC PDOX models regarding the association between CTC characteristics and distant metastasis following excision of the primary tumor xenograft. This study assembles a timeline of PDOX tumor shedding and metastatic tumor progression before and after tumor excision surgery. We report the ability to use tumorectomies to increase the lifespan of TNBC PDOX models with the potential to obtain larger metastases. CTC clusters and CTCs expressing a mesenchymal marker (vimentin) were associated with metastatic burden in lung and liver. The data collected through these experiments will guide the further use of PDOX models in studying metastatic TNBC.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V et al (2011) Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol 24(2):157–167
Haffty BG, Yang QF, Reiss M, Kearney T, Higgins SA, Weidhaas J et al (2006) Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 24(36):5652–5657
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15):4429–4434
Rakha EA, El-Sayed ME, Green AR, Lee AHS, Robertson JF, Ellis IO (2007) Prognostic markers in triple-negative breast cancer. Cancer 109(1):25–32
Sharma P, López-Tarruella S, García-Saenz JA, Khan QJ, Gómez HL, Prat A et al (2018) Pathological response and survival in triple-negative breast cancer following neoadjuvant carboplatin plus docetaxel. Clin Cancer Res 24(23):5820–5829
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26(8):1275–1281
Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277
Nguyen DX, Bos PD, Massague J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9(4):274–284
Morton CL, Houghton PJ (2007) Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc 2(2):247–50
Bosma MJ, Carroll AM (1991) The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev of Immunol 9:323–50
Manzotti C, Audisio RA, Pratesi G (1993) Importance of orthotopic implantation for human tumors as model systems: relevance to metastasis and invasion. Clin Exp Metastasis 11(1):5–14
Rygaard J, Povlsen CO (2007) Heterotransplantation of a human malignant tumour to “Nude” mice. Apmis 115(5):604–6
Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen XH, Chaleff S et al (2005) Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma(null) mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174(10):6477–89
Simpson-Abelson MR, Sonnenberg GF, Takita H, Yokota SJ, Conway TF, Kelleher RJ et al (2008) Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2R gamma(null) mice. J Immunol 180(10):7009–18
DeRose YS, Wang GY, Lin YC, Bernard PS, Buys SS, Ebbert MTW et al (2011) Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 17(11):1514–1520
Iorns E, Drews-Elger K, Ward TM, Dean S, Clarke J, Berry D et al (2012) A new mouse model for the study of human breast cancer metastasis. PLoS ONE 7(10):e47995
Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C et al (2014) Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 4(9):998–1013
Jin KT, Teng LS, Shen YP, He KF, Xu ZZ, Li GL (2010) Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clin Transl Oncol 12(7):473–80
Hoffman RM (2015) Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer 15(8):451–2
Hoffman RM (1999) Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs 17(4):343–59
Fu X, Le P, Hoffman RM (1993) A metastatic orthotopic-transplant nude-mouse model of human patient breast cancer. Anticancer Res 13(4):901–4
Eccles SA (2011) Models for evaluation of targeted therapies of invasive and metastatic disease. In: Teicher B (ed) Tumor models in cancer research. Cancer Drug Discovery and Development. Humana Press, Totowa, NJ, pp 447–495
Kawaguchi T, Foster BA, Young J, Takabe K (2017) Current update of patient-derived xenograft model for translational breast cancer research. J Mammary Gland Biol Neoplasia 22(2):131–9
Zhang HY, Cohen AL, Krishnakumar S, Wapnir IL, Veeriah S, Deng G et al (2014) Patient-derived xenografts of triple-negative breast cancer reproduce molecular features of patient tumors and respond to mTOR inhibition. Breast Cancer Res 16(2):R36
Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang XF, Iacobuzio-Donahue C, Karikari C et al (2006) An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res 12(15):4652–61
Kanaya N, Somlo G, Wu J, Frankel P, Kai M, Liu XL et al (2017) Characterization of patient-derived tumor xenografts (PDXs) as models for estrogen receptor positive (ER+HER2- and ER+HER2+) breast cancers. J Steroid Biochem Mol Biol 170:65–74
Bibby MC (2004) Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer 40(6):852–7
Gast CE, Shaw AK, Wong MH, Coussens LM (2017) Surgical procedures and methodology for a preclinical murine model of de novo mammary cancer metastasis. J Vis Exp (125).
Paez-Ribes M, Man S, Xu P, Kerbel RS (2016) Development of patient derived xenograft models of overt spontaneous breast cancer metastasis: a cautionary note. PLoS ONE 11(6):e0158034
Cristofanilli M (2006) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol 33(3):S9–S14
Sethi N, Kang YB (2011) Unravelling the complexity of metastasis - molecular understanding and targeted therapies. Nat Rev Cancer 11(10):735–48
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C et al (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10(20):6897–904
Hayes DF, Smerage J (2008) Is there a role for circulating tumor cells in the management of breast cancer? Clin Cancer Res 14(12):3646–50
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC et al (2006) Circulating tumor cells versus imaging - predicting overall survival in metastatic breast cancer. Clin Cancer Res 12(21):6403–9
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–74
Guarino M, Rubino B, Ballabio G (2007) The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39(3):305–18
Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED et al (2007) Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol 213(2):374–83
Ramani VC, Lemaire CA, Triboulet M, Casey KM, Heirich K, Renier C et al (2019) Investigating circulating tumor cells and distant metastases in patient-derived orthotopic xenograft models of triple-negative breast cancer. Breast Cancer Res 21(1):98
Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S (2011) Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res 13(3):R59
Polioudaki H, Agelaki S, Chiotaki R, Politaki E, Mavroudis D, Matikas A et al (2015) Variable expression levels of keratin and vimentin reveal differential EMT status of circulating tumor cells and correlation with clinical characteristics and outcome of patients with metastatic breast cancer. BMC Cancer 15:399
Lowes LE, Allan AL (2018) Circulating tumor cells and implications of the epithelial-to-mesenchymal transition. Adv Clin Chem 83:121–81
Klein CA (2003) The systemic progression of human cancer: a focus on the individual disseminated cancer cell - the unit of selection. Adv Cancer Res 89:35–67
Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V et al (2013) Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 31(6):539–544
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA et al (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158(5):1110–22
Giuliano M, Herrera S, Christiny P, Shaw C, Creighton CJ, Mitchell T et al (2015) Circulating and disseminated tumor cells from breast cancer patient-derived xenograft-bearing mice as a novel model to study metastasis. Breast Cancer Res 17:3
Larsson AM, Jansson S, Bendahl PO, Jorgensen CLT, Loman N, Graffman C et al (2018) Longitudinal enumeration and cluster evaluation of circulating tumor cells improve prognostication for patients with newly diagnosed metastatic breast cancer in a prospective observational trial. Breast Cancer Res 20(1):48
Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN et al (2016) Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci USA 113(7):E854–E863
Suo YZ, Xie CY, Zhu X, Fan ZC, Yang ZR, He H et al (2017) Proportion of circulating tumor cell clusters increases during cancer metastasis. Cytometry A 91(3):250–253
Yu M, Bardia A, Wittner B, Stott SL, Smas ME, Ting DT et al (2013) Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339(6119):580–4
Zajchowski DA, Bartholdi MF, Gong Y, Webster L, Liu HL, Munishkin A et al (2001) Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res 61(13):5168–78
Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XHF, Norton L et al (2009) Tumor self-seeding by circulating cancer cells. Cell 139(7):1315–26
Demicheli R (2001) Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Semin Cancer Biol 11(4):297–305
Doornebal CW, Klarenbeek S, Braumuller TM, Klijn CN, Ciampricotti M, Hau CS et al (2013) A preclinical mouse model of invasive lobular breast cancer metastasis. Cancer Res 73(1):353–63
Keller PJ, Lin AF, Arendt LM, Klebba I, Jones AD, Rudnick JA et al (2010) Mapping the cellular and molecular heterogeneity of normal and malignant breast tissues and cultured cell lines. Breast Cancer Res. 12(5):R87
Dominici LS, Mittendorf EA, Wang XM, Liu J, Kuerer HM, Hunt KK et al (2012) Implications of constructed biologic subtype and its relationship to locoregional recurrence following mastectomy. Breast Cancer Res. 14(3):R82
Pistelli M, Pagliacci A, Battelli N, Santinelli A, Biscotti T, Ballatore Z et al (2013) Prognostic factors in early-stage triple-negative breast cancer: lessons and limits from clinical practice. Anticancer Res 33(6):2737–42
Mohammed RAA, Ellis IO, Mahmmod AM, Hawkes EC, Green AR, Rakha EA et al (2011) Lymphatic and blood vessels in basal and triple-negative breast cancers: characteristics and prognostic significance. Mod Pathol 24(6):774–85
Ugras S, Stempel M, Patil S, Morrow M (2014) Estrogen receptor, progesterone receptor, and HER2 status predict lymphovascular invasion and lymph node involvement. Ann Surg Oncol 21(12):3780–6
Coughlan AM, Harmon C, Whelan S, O'Brien EC, O'Reilly VP, Crotty P et al (2016) Myeloid engraftment in humanized mice: impact of granulocyte-colony stimulating factor treatment and transgenic mouse strain. Stem Cells Dev 25(7):530–41
Noel A, Depauwgillet MC, Purnell G, Nusgens B, Lapiere CM, Foidart JM (1993) Enhancement of tumorigenicity of human breast adenocarcinoma cells in nude mice by matrigel and fibroblasts. Br J Cancer 68(5):909–15
Fridman R, Kibbey MC, Royce LS, Zain M, Sweeney TM, Jicha DL et al (1991) Enhanced tumor-growth of both primary and established human and murine tumor-cells in athymic mice after coinjection with matrigel. J Natl Cancer Inst 83(11):769–74
Lemaire CA, Liu SZ, Wilkerson CL, Ramani VC, Barzanian NA, Huang KW et al (2018) Fast and label-free isolation of circulating tumor cells from blood: from a research microfluidic platform to an automated fluidic instrument, VTX-1 liquid biopsy system. Slas Technol 23(1):16–29
Che J, Yu V, Dhar M, Renier C, Matsumoto M, Heirich K et al (2016) Classification of large circulating tumor cells isolated with ultra-high throughput microfluidic Vortex technology. Oncotarget 7(11):12748–60
Acknowledgements
The authors would like to thank the Department of Comparative Medicine, the Master of Laboratory Animal Science (MLAS) Training Program, and the Veterinary Service Center (VSC) at Stanford University for their support of this project. We would especially like to acknowledge and thank Elias Godoy of the VSC for his assistance. This project was funded through the Stanford MLAS Graduate Student Fund (AMR), the John and Marva Warnock Research Fund (SSJ), and NIH grants 4P30CA124435 that supports the Biostatistics Core of the Stanford Cancer Institute (AM) and SPECTRUM award number 1UL1TR003142 (AM).
Author information
Authors and Affiliations
Contributions
AMR, ES, GNK, SWB, VCR, SSJ, and KMC designed and planned the experiments. AMR, GNK, SWB, MBB, CAL, and VCR performed the experiments; AMR and KMC performed histologic studies. AMR, AM, SSJ, and KMC analyzed the data. AMR wrote the manuscript with assistance from the other authors. All authors have reviewed and approved this manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
CAL and ES have financial interests in Vortex Biosciences and intellectual property described herein. SSJ serves as an expert advisor for Ravel Biotechnology, which is developing an analytic platform for early cancer detection using cell-free DNA. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Razmara, A.M., Sollier, E., Kisirkoi, G.N. et al. Tumor shedding and metastatic progression after tumor excision in patient-derived orthotopic xenograft models of triple-negative breast cancer. Clin Exp Metastasis 37, 413–424 (2020). https://doi.org/10.1007/s10585-020-10033-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-020-10033-3