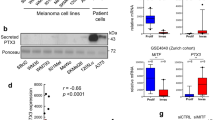
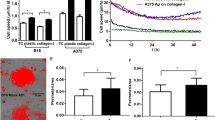
Abstract
Metastatic melanoma is one of the most aggressive forms of skin cancer and has a poor prognosis. We have previously identified Annexin A1 (ANXA1) as a potential murine melanoma-spreading factor that may modulate cell invasion by binding to formyl peptide receptors (FPRs). Here, we report that (1) in a B16Bl6 spontaneous metastasis model, a siRNA-induced decrease in tumoral ANXA1 expression significantly reduced tumoral MMP2 activity and number of lung metastases; (2) in a retrospective study of 61 patients, metastasis-free survival was inversely related to ANXA1 expression levels in primary tumors (HR 3.15 [1.03–9.69], p = 0.045); (3) in human melanoma cell lines, ANXA1 level was positively correlated with in vitro invasion capacity whereas normal melanocytes contained low ANXA1 levels, and (4) the ANXA1 N-terminal peptide ANXA12–26 stimulated MMP2 activity after interaction with FPRs and significantly stimulated the in vitro invasion of melanomas by acting on FPRs. These findings identify ANXA1 as a proinvasive protein in melanoma that holds promise as a potential prognostic marker and therapeutic target.





Similar content being viewed by others
References
Chiang AC, Massague J (2008) Molecular basis of metastasis. N Engl J Med 359(26):2814–2823
Melnikova VO, Bar-Eli M (2009) Inflammation and melanoma metastasis. Pigment Cell Melanoma Res 22(3):257–267
Orgaz JL, Sanz-Moreno V (2012) Emerging molecular targets in melanoma invasion and metastasis. Pigment Cell Melanoma Res 26(1):39–57
Davies H et al (2002) Mutations of the BRAF gene in human cancer. Nature 417(6892):949–954
Bollag G et al (2010) Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 467(7315):596–599
Chapman PB et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364(26):2507–2516
Heidorn SJ et al (2011) Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140(2):209–221
Poulikakos PI et al (2011) RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 480(7377):387–390
Rondepierre F et al (2009) Proteomic studies of B16 lines: involvement of annexin A1 in melanoma dissemination. Biochim Biophys Acta 1794(1):61–69
Parente L, Solito E (2004) Annexin 1: more than an anti-phospholipase protein. Inflamm Res 53(4):125–132
Lim LH, Pervaiz S (2007) Annexin 1: the new face of an old molecule. FASEB J 21(4):968–975
Mussunoor S, Murray GI (2008) The role of annexins in tumour development and progression. J Pathol 216(2):131–140
Li CF et al (2010) Annexin-I overexpression is associated with tumour progression and independently predicts inferior disease-specific and metastasis-free survival in urinary bladder urothelial carcinoma. Pathology 42(1):43–49
Schittenhelm J et al (2009) Comparative analysis of annexin-1 in neuroepithelial tumors shows altered expression with the grade of malignancy but is not associated with survival. Mod Pathol 22(12):1600–1611
Schetter AJ et al (2009) Association of inflammation-related and microRNA gene expression with cancer-specific mortality of colon adenocarcinoma. Clin Cancer Res 15(18):5878–5887
Nan Y et al (2009) Analysis of the expression protein profiles of lung squamous carcinoma cell using shot-gun proteomics strategy. Med Oncol 26(2):215–221
Inokuchi J et al (2009) Loss of annexin A1 disrupts normal prostate glandular structure by inducing autocrine IL-6 signaling. Carcinogenesis 30(7):1082–1088
Nomura H et al (2009) Down-regulation of plasma membranous Annexin A1 protein expression in premalignant and malignant lesions of the oral cavity: correlation with epithelial differentiation. J Cancer Res Clin Oncol 135(7):943–949
Yu G et al (2008) Tissue microarray analysis reveals strong clinical evidence for a close association between loss of annexin A1 expression and nodal metastasis in gastric cancer. Clin Exp Metastasis 25(7):695–702
Geary LA et al (2014) CAF-secreted annexin A1 induces prostate cancer cells to gain stem cell-like features. Mol Cancer Res 12(4):607–621
Perretti M, D’Acquisto F (2009) Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol 9(1):62–70
Babbin BA et al (2006) Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem 281(28):19588–19599
de Graauw M et al (2011) Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sci USA 107(14):6340–6345
Maschler S et al (2010) Annexin A1 attenuates EMT and metastatic potential in breast cancer. EMBO Mol Med 2(10):401–414
Wang LP et al (2010) Annexin A1 expression and its prognostic significance in human breast cancer. Neoplasma 57(3):253–259
Cao Y et al (2008) Loss of annexin A1 expression in breast cancer progression. Appl Immunohistochem Mol Morphol 16(6):530–534
Kamal AM, Flower RJ, Perretti M (2005) An overview of the effects of annexin 1 on cells involved in the inflammatory process. Mem Inst Oswaldo Cruz 100(Suppl 1):39–47
Le Y, Murphy PM, Wang JM (2002) Formyl-peptide receptors revisited. Trends Immunol 23(11):541–548
Bena S et al (2012) Annexin A1 interaction with the FPR2/ALX receptor: identification of distinct domains and downstream associated signaling. J Biol Chem 287(29):24690–24697
Ye RD et al (2009) International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev 61(2):119–161
Zhou Y et al (2005) Formylpeptide receptor FPR and the rapid growth of malignant human gliomas. J Natl Cancer Inst 97(11):823–835
Huang J et al (2008) Receptor “hijacking” by malignant glioma cells: a tactic for tumor progression. Cancer Lett 267(2):254–261
Chakravarti N et al (2012) Differential expression of the G-protein-coupled formyl peptide receptor in melanoma associates with aggressive phenotype. Am J Dermatopathol 35(2):184–190
Bonnet M et al (2010) Anti-melanoma efficacy of internal radionuclide therapy in relation to melanin target distribution. Pigment Cell Melanoma Res 23(5):e1–e11
Duncan R et al (2008) Characterisation and protein expression profiling of annexins in colorectal cancer. Br J Cancer 98(2):426–433
Bai XF et al (2004) Overexpression of annexin 1 in pancreatic cancer and its clinical significance. World J Gastroenterol 10(10):1466–1470
Malech HL et al (1985) Asparagine-linked oligosaccharides on formyl peptide chemotactic receptors of human phagocytic cells. J Biol Chem 260(4):2509–2514
Hoek KS, Goding CR (2010) Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res 23(6):746–759
Cheli Y et al (2011) Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene 30(20):2307–2318
Yi M, Schnitzer JE (2009) Impaired tumor growth, metastasis, angiogenesis and wound healing in annexin A1-null mice. Proc Natl Acad Sci USA 106(42):17886–17891
Kang JS et al (2002) Dysregulation of annexin I protein expression in high-grade prostatic intraepithelial neoplasia and prostate cancer. Clin Cancer Res 8(1):117–123
Sawmynaden P, Perretti M (2006) Glucocorticoid upregulation of the annexin-A1 receptor in leukocytes. Biochem Biophys Res Commun 349(4):1351–1355
Hashimoto A et al (2007) Glucocorticoids co-interact with lipoxin A4 via lipoxin A4 receptor (ALX) up-regulation. Biomed Pharmacother 61(1):81–85
Hayhoe RP et al (2006) Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood 107(5):2123–2130
Naba A et al (2012) The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 11(4):M111.014647
Gavins FN, Hickey MJ (2012) Annexin A1 and the regulation of innate and adaptive immunity. Front Immunol 3:354
Fridman WH et al (2014) The immune microenvironment: a major player in human cancers. Int Arch Allergy Immunol 164(1):13–26
Lines JL et al (2014) VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res 2(6):510–517
D’Acquisto F et al (2007) Annexin-1 modulates T-cell activation and differentiation. Blood 109(3):1095–1102
Cooray SN et al (2013) Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci USA 110(45):18232–18237
Li Y, Ye D (2013) Molecular biology for formyl peptide receptors in human diseases. J Mol Med (Berl) 91(7):781–789
Xie TX et al (2004) Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 23(20):3550–3560
Schnaeker EM et al (2004) Microtubule-dependent matrix metalloproteinase-2/matrix metalloproteinase-9 exocytosis: prerequisite in human melanoma cell invasion. Cancer Res 64(24):8924–8931
Gorzelanny C et al (2007) Specific interaction between chitosan and matrix metalloprotease 2 decreases the invasive activity of human melanoma cells. Biomacromolecules 8(10):3035–3040
Vaisanen A et al (1998) Prognostic value of MMP-2 immunoreactive protein (72 kD type IV collagenase) in primary skin melanoma. J Pathol 186(1):51–58
Gould Rothberg BE, Bracken MB, Rimm DL (2009) Tissue biomarkers for prognosis in cutaneous melanoma: a systematic review and meta-analysis. J Natl Cancer Inst 101(7):452–474
Redondo P et al (2005) Expression and serum levels of MMP-2 and MMP-9 during human melanoma progression. Clin Exp Dermatol 30:541–545
Acknowledgments
We are grateful to Dr François Boulay (UMR5092, CEA Grenoble) for gifting the FPR HL-60 cells and to Laurie Joumard for her participation in the molecular characterization of melanoma cell lines for FPR status. This work was supported by the Ligue Régionale Contre le Cancer and the Société Française de Dermatologie. ZB received fellowship support via a Conseil Régional d’Auvergne-backed ‘Innovative funds’ project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zied Boudhraa and Fabien Rondepierre have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2014_9665_MOESM1_ESM.pdf
300 pmol ANXA1 and control siRNA were injected at days 11, 14 and 18 into B16Bl6 tumors obtained after grafting 3x105 cells into C57BL6 mice. For each treatment, 20 mice were sacrificed at day 23. Proteins were extracted from each tumor. qPCR and western blotting analyses revealed a significant decrease in steady-state levels of ANXA1 and mRNA protein levels, respectively, as quantified by the ANXA1/26S and ANXA1/GAPDH ratios (* Student’s t-test, p<0.05). a.u. = arbitrary units. Supplementary material 1 (PDF 35 kb)
Rights and permissions
About this article
Cite this article
Boudhraa, Z., Rondepierre, F., Ouchchane, L. et al. Annexin A1 in primary tumors promotes melanoma dissemination. Clin Exp Metastasis 31, 749–760 (2014). https://doi.org/10.1007/s10585-014-9665-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-014-9665-2