Abstract
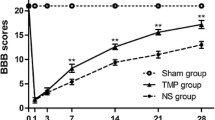
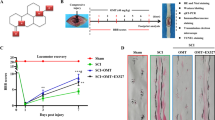
Hyperbaric oxygen preconditioning (HBO-PC) has beneficial effects on the promotion of locomotor recovery by reducing apoptosis and inflammation after traumatic spinal cord injury (SCI). The mammalian target of rapamycin (mTOR) signaling pathway has been implicated in apoptosis and inflammation in many pathophysiological conditions. However, whether HBO-PC improves traumatic SCI-induced locomotor dysfunction by regulating the mTOR signaling pathway and its downstream molecules remains unknown. In the present study, we found that HBO-PC significantly promoted SCI-induced hind-limb locomotor recovery and increased the amplitude and potential of motor evoked potential. Magnetic resonance imaging showed that spinal cavitation or atrophy caused by SCI was obviously alleviated by HBO-PC therapy. Histological analysis showed that the changes in spinal cord neural structure in SCI rats were markedly restored by HBO-PC treatment. Western blot analysis showed that the SCI-induced enhanced levels of p-mTOR, inflammatory cytokines and apoptosis in the spinal cord were abrogated after administration of HBO-PC. Furthermore, intrathecal administration of an mTOR agonist reversed the effects of HBO-PC on locomotor function recovery, p-NF-κB p65 and p-p70S6K levels, inflammation and apoptosis. These findings indicated a new mechanism by which HBO-PC therapy suppressed inflammation and apoptosis through inactivation of the mTOR signaling pathway, which contributed to motor disability in SCI rats.







Similar content being viewed by others
References
Ahuja CS et al (2017) Traumatic spinal cord injury. Nat Rev Dis Primers 3:17018
Allen AR (1911) Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column: a preliminary report. J Am Med Assoc LVII(11):878–880
Allison DJ, Ditor DS (2015) Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord 53(1):14–18
Berry M et al (2016) Prospects for mTOR-mediated functional repair after central nervous system trauma. Neurobiol Dis 85:99–110
Duan X et al (2015) Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 85(6):1244–1256
Emery E et al (1998) Apoptosis after traumatic human spinal cord injury. J Neurosurg 89(6):911–920
Erlich S et al (2007) Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis 26(1):86–93
Ghosh A et al (2016) Mitochondrial pyruvate carrier regulates autophagy, inflammation, and neurodegeneration in experimental models of Parkinson’s disease. Sci Transl Med 8(368):368ra174
Goldshmit Y et al (2015) Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci 68:82–91
Haltern C et al (2000) Hyperbaric oxygen therapy (HBO): current standing. Anasthesiol Intensivmed Notfallmed Schmerzther 35(8):487–502
He B, Nan G (2016) Neuronal regeneration after acute spinal cord injury in adult rats. Spine J 16(12):1459–1467
Huang L et al (2003) The role of multiple hyperbaric oxygenation in expanding therapeutic windows after acute spinal cord injury in rats. J Neurosurg 99(2 Suppl):198–205
Jain NB et al (2015) Traumatic spinal cord injury in the United States, 1993–2012. JAMA 313(22):2236–2243
Kwon BK et al (2004) Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 4(4):451–464
Lin WP et al (2016) Effect of adenovirus-mediated RNA interference of IL-1beta expression on spinal cord injury in rats. Spinal Cord 54(10):778–784
Liu X et al (2015) Hyperbaric oxygen treatment protects against spinal cord injury by inhibiting endoplasmic reticulum stress in rats. Spine (Phila Pa 1976) 40(24):E1276–E1283
Ma T et al (2010) Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer’s disease. PLoS ONE 5(9):e12845
Meng L et al (2018) The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-kappaB and PI3K/Akt/mTOR pathways. Mol Immunol 94:7–17
Morris G et al (2019) Could Alzheimer’s disease originate in the periphery and if so how so? Mol Neurobiol 56(1):406–434
Orr MB, Gensel JC (2018) Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 15(3):541–553
Patel NP, Huang JH (2017) Hyperbaric oxygen therapy of spinal cord injury. Med Gas Res 7(2):133–143
Pita-Thomas W et al (2019) HDAC5 promotes optic nerve regeneration by activating the mTOR pathway. Exp Neurol 317:271–283
Rogers WK, Todd M (2016) Acute spinal cord injury. Best Pract Res Clin Anaesthesiol 30(1):27–39
Sekiguchi A et al (2012) Rapamycin promotes autophagy and reduces neural tissue damage and locomotor impairment after spinal cord injury in mice. J Neurotrauma 29(5):946–956
Sharma HS et al (2003) Topical application of TNF-alpha antiserum attenuates spinal cord trauma induced edema formation, microvascular permeability disturbances and cell injury in the rat. Acta Neurochir Suppl 86:407–413
Sun SC (2017) The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol 17(9):545–558
Tai PA et al (2010) Attenuating experimental spinal cord injury by hyperbaric oxygen: stimulating production of vasculoendothelial and glial cell line-derived neurotrophic growth factors and interleukin-10. J Neurotrauma 27(6):1121–1127
Tan AM et al (2008) Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury. J Neurosci 28(49):13173–13183
Temiz-Resitoglu M et al (2017) Activation of mTOR/IkappaB-alpha/NF-kappaB pathway contributes to LPS-induced hypotension and inflammation in rats. Eur J Pharmacol 802:7–19
Wang L et al (2009) Hyperbaric oxygen preconditioning attenuates early apoptosis after spinal cord ischemia in rats. J Neurotrauma 26(1):55–66
Winden KD, Ebrahimi-Fakhari D, Sahin M (2018) Abnormal mTOR activation in autism. Annu Rev Neurosci 41:1–23
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124(3):471–484
Yu JS, Cui W (2016) Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143(17):3050–3060
Zhai X et al (2016) Hyperbaric oxygen preconditioning ameliorates hypoxia–ischemia brain damage by activating Nrf2 expression in vivo and in vitro. Free Radic Res 50(4):454–466
Zhou M et al (2018) Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-kappaB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine 35:345–360
Funding
The study was supported by the Natural Science Foundation of Guangdong Province (No. 2017A030313693), the Research Program of PLA (No. CGZ16C004) and the Military Logistics Research Project (CN) (No. CWH17J022).
Author information
Authors and Affiliations
Contributions
HC and YZ designed the study, and HC, GX, YW, XW and FW performed the experiments. HC and GX analyzed the data, and YZ wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Ethical Approval
The experiments were performed in accordance with the guidelines of the General Hospital of Southern Theater Command Animal Ethics Committee. The protocol number is SYXK (Yue) 2019-0100.
Informed Consent
All authors provided consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Xu, G., Wu, Y. et al. HBO-PC Promotes Locomotor Recovery by Reducing Apoptosis and Inflammation in SCI Rats: The Role of the mTOR Signaling Pathway. Cell Mol Neurobiol 41, 1537–1547 (2021). https://doi.org/10.1007/s10571-020-00921-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00921-3