Abstract
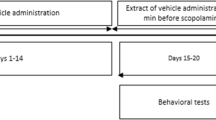
Vitellaria paradoxa C.F. Gaertn (Sapotaceae) is a perennial three which naturally grows in the northern part of Cameroon. It has been traditionally used in the Cameroonian folk medicine for treating inflammation and pain. In the present study, we evaluate the possible anti-amnesic and antioxidative effects of the methanolic extract of V. paradoxa stem bark in an Alzheimer’s disease (AD) rat model of scopolamine. Rats received a single injection of scopolamine (1.5 mg/kg) before behavioral testing and were treated with the methanolic extract (25 and 50 mg/kg), daily, for eight continuous days. Also, the antioxidant activity in the hippocampus was assessed using the total content of reduced glutathione and malondialdehyde levels. The scopolamine-treated rats exhibited the following: decrease of exploratory time and discrimination index within the novel object recognition test, decrease of spontaneous alternations percentage within Y-maze task, and increase of working memory errors, reference memory errors, and time taken to consume all five baits within radial arm-maze task. Administration of the methanolic extract significantly improved these parameters, suggesting positive effects on memory formation processes and antioxidant potential. Our results suggest that the methanolic extract ameliorates scopolamine-induced memory impairment by attenuation of the oxidative stress in the rat hippocampus.






Similar content being viewed by others
References
Aboaba S, Akande A, Flamini G (2014) Chemical composition and toxicity of essential oil of Vitellaria paradoxa (C.F. Gaertn.) from Nigeria. J Essent Oil Bear Plants 17(1):126–130. doi:10.1080/0972060X.2014.884757
Ahmed F, Ghalib RM, Sasikala P, Ahmed KKM (2013) Cholinesterase inhibitors from botanicals. Pharmacogn Rev 7(14):121–130. doi:10.4103/0973-7847.120511
Ayankunle AA, Kolawole O, Adesokan A, Akiibinu M (2012) Antibacterial activity and sub-chronic toxicity studies of Vitellaria paradoxa stem bark extract. J Pharmacol Toxicol 7:298–304
Bartus R, Dean RL 3rd, Sherman KA, Friedman E, Beer B (1981) Profound effects of combining choline and piracetam on memory enhancement and cholinergic function in aged rats. Neurobiol Aging 2:105–111
Beppe GJ, Dongmo AB, Foyet HS, Tsabang N, Olteanu Z, Cioanca O, Hancianu M, Dimo T, Hritcu L (2014) Memory-enhancing activities of the aqueous extract of Albizia adianthifolia leaves in the 6-hydroxydopamine-lesion rodent model of Parkinson’s disease. BMC Complement Altern Med 14:142. doi:10.1186/1472-6882-14-142
Coppede F, Migliore L (2009) DNA damage and repair in Alzheimer’s disease. Curr Alzheimer Res 6:36–47
Drever B, Anderson W, Johnson H, O’Callaghan M, Seo S, Choi D-Y, Riedel G, Platt B (2007) Memantine acts as a cholinergic stimulant in the mouse hippocampus. J Alzheimers Dis 12:319–333
Dumont M, Beal MF (2011) Neuroprotective strategies involving ROS in Alzheimer’s disease. Free Radic Biol Med 51(5):1014–1026. doi:10.1016/j.freeradbiomed.2010.11.026
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31:47–59
Eyong K, Foyet H, Baïry G, Folefoc GN, Asongalem EA, Lagojda A, Lamshöft M (2015) A new ursane triterpenoic acid and other potential anti-inflammatory and anti-arthritic constituents from EtOAc extracts of Vitellaria paradoxa stem bark. J Ethnopharmacol 174(4):277–286. doi:10.1016/j.jep.2015.08.014
Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J, Ding J, Geng M (2005) Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett 374(3):222–226. doi:10.1016/j.neulet.2004.10.063
Ferreiro E, Baldeiras I, Ferreira IL, Costa RO, Rego AC, Pereira CF, Oliveira CR (2012) Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer’s disease: from pathogenesis to biomarkers. Int J Cell Biol 2012:735206. doi:10.1155/2012/735206
Foyet HS, Hritcu L, Ciobica A, Stefan M, Kamtchouing P, Cojocaru D (2011) Methanolic extract of Hibiscus asper leaves improves spatial memory deficits in the 6-hydroxydopamine-lesion rodent model of Parkinson’s disease. J Ethnopharmacol 133:773–779
Foyet H, Tsala D, Ngatanko Abaissou H (2014) Enhancing spatial memory: anxiolytic and antidepressant effects of Tapinanthus dodoneifolius (DC) Danser in mice. Neurol Res Int 2014:974308. doi:10.1155/2014/974308
Fukuzawa K, Tokumura A (1976) Glutathione peroxidase activity in tissues of vitamin E-deficient mice. J Nutr Sci Vitaminol (Tokyo) 22:405–407
Gawande DY, Goel RK (2015) Pharmacological validation of in silico guided novel nootropic potential of Achyranthes aspera L. J Ethnopharmacol 175:324–334. doi:10.1016/j.jep.2015.09.025
Goverdhan P, Sravanthi A, Mamatha T (2012) Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int J Alzheimers Dis 2012:974013. doi:10.1155/2012/974013
Gupta S, Garg GR, Bharal N, Mediratta PK, Banerjee BD, Sharma KK (2009) Reversal of propoxur-induced impairment of step-down passive avoidance, transfer latency and oxidative stress by piracetam and ascorbic acid in rats. Environ Toxicol Pharmacol 28(3):403–408. doi:10.1016/j.etap.2009.06.007
He M, Zhao L, Wei M-J, Yao W-F, Zhao H-S, Chen F-J (2009) Neuroprotective effects of (−)-Epigallocatechin-3-gallate on aging mice induced by D-Galactose. Biol Pharm Bull 32(1):55–60. doi:10.1248/bpb.32.55
Hritcu L, Nabeshima T (2009) Kainic acid lesion-induced spatial memory deficits of rats. Cent Eur J Biol 4:179–185
Hritcu L, Cioanca O, Hancianu M (2012) Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 19(6):529–534. doi:10.1016/j.phymed.2012.02.002
Hritcu L, Noumedem J, Cioanca O, Hancianu M, Kuete V, Mihasan M (2014) Methanolic extract of Piper nigrum fruits improves memory impairment by decreasing brain oxidative stress in amyloid beta(1–42) rat model of Alzheimer’s Disease. Cell Mol Neurobiol 34(3):437–449. doi:10.1007/s10571-014-0028-y
Ibrahim A (2013) Effects of Edaravone on scopolamine induced-dementia in experimental rats. Int J Pharmacol 9:271–276
Ischiropoulos H, Beckman JS (2003) Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Investig 111(2):163–169. doi:10.1172/JCI200317638
Jawaid T, Jahan S, Kamal M (2015) A comparative study of neuroprotective effect of angiotensin converting enzyme inhibitors against scopolamine-induced memory impairments in rats. J Adv Pharm Technol Res 6(3):130–135. doi:10.4103/2231-4040.161514
Jiang S, Li Y, Zhang C, Zhao Y, Bu G, Xu H, Zhang Y-W (2014) M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neurosci Bull 30(2):295–307. doi:10.1007/s12264-013-1406-z
Lee M-R, Yun B-S, Park S-Y, Ly S-Y, Kim S-N, Han B-H, Sung C-K (2010) Anti-amnesic effect of Chong–Myung–Tang on scopolamine-induced memory impairments in mice. J Ethnopharmacol 132(1):70–74. doi:10.1016/j.jep.2010.07.041
Liu M, Chen F, Sha L, Wang S, Tao L, Yao L, He M, Yao Z, Liu H, Zhu Z, Zhang Z, Zheng Z, Sha X, Wei M (2014) (−)-Epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75NTR signaling in APP/PS1 transgenic mice. Mol Neurobiol 49(3):1350–1363. doi:10.1007/s12035-013-8608-2
Lombardo S, Maskos U (2015) Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 96(Part B 0):255–262. doi:10.1016/j.neuropharm.2014.11.018
Luque-Contreras D, Carvajal K, Toral-Rios D, Franco-Bocanegra D, Campos-Peña V (2014) Oxidative stress and Metabolic Syndrome: cause or consequence of Alzheimer’s Disease? Oxid Med Cell Longev 2014:497802. doi:10.1155/2014/497802
Markesbery WR, Lovell MA (1998) Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s Disease. Neurobiol Aging 19(1):33–36. doi:10.1016/S0197-4580(98)00009-8
Matig OE, Ndoye O, Kengue J, Awono A (2006) Les fruitiers forestiers comestibles du Cameroun. IPGRI Regional Office for West and Central Africa, 150–152
Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G (2008) Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med 44(8):1493–1505. doi:10.1016/j.freeradbiomed.2008.01.002
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pardo Andreu GL, Maurmann N, Reolon GK, de Farias CB, Schwartsmann G, Delgado R, Roesler R (2010) Mangiferin, a naturally occurring glucoxilxanthone improves long-term object recognition memory in rats. Eur J Pharmacol 635(1–3):124–128. doi:10.1016/j.ejphar.2010.03.011
Peña Id, Yoon SY, Kim HJ, Park S, Hong EY, Ryu JH, Park IH, Cheong JH (2014) Effects of ginseol k-g3, an Rg3-enriched fraction, on scopolamine-induced memory impairment and learning deficit in mice. J Ginseng Res 38(1):1–7. doi:10.1016/j.jgr.2013.11.003
Sanou H, Lamien N (2011) Vitellaria paradoxa, karité: Conservation et utilisation durable des ressources génétiques des espèces ligneuses alimentaires prioritaires de l’Afrique subsaharienne. Saforgen:2–4
Teixeira MDA, Souza CM, Menezes APF, Carmo MRS, Fonteles AA, Gurgel JP, Lima FAV, Viana GSB, Andrade GM (2013) Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol Biochem Behav 110:1–7. doi:10.1016/j.pbb.2013.05.012
Yang MH, Yoon KD, Chin Y-W, Park JH, Kim SH, Kim YC, Kim J (2009) Neuroprotective effects of Dioscorea opposita on scopolamine-induced memory impairment in in vivo behavioral tests and in vitro assays. J Ethnopharmacol 121(1):130–134. doi:10.1016/j.jep.2008.10.010
Ziba L, Yameogo F (2002) Les bienfaits du karité pour les populations rurales, les communautés et les pays. Actes de l’atelier organisé par l’ONU pour l’Alimentation et l’Agriculture, le Fonds commun pour les Produits de Base et le Centre de Suivi Ecologique. 80
Acknowledgments
The authors are grateful to Mr. Jean Paul Bamba from Fauna School of Garoua (Cameroon) for providing the plant location and identification.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors have declared that no competing interests exist.
Rights and permissions
About this article
Cite this article
Foyet, H.S., Asongalem, A.E., Oben, E.K. et al. Effects of the Methanolic Extract of Vitellaria paradoxa Stem Bark Against Scopolamine-Induced Cognitive Dysfunction and Oxidative Stress in the Rat Hippocampus. Cell Mol Neurobiol 36, 1139–1149 (2016). https://doi.org/10.1007/s10571-015-0310-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0310-7