Abstract
Owing to unique physiochemical and biological properties as well as the ability to be combined with a wide variety of materials for both biocompatibility and hydrophilia, carboxymethyl cellulose (CMC) is an excellent choice as a carrier. Loading Chlorine dioxide (ClO2) into biodegradable carrier for its good disinfection performance and high safety factors has attracted significantattention. Therefore, in this study, we used ClO2 as a model drug, and a sustained-ClO2-gas-release gel was developed from degradable materials, such as carboxymethyl cellulose (CMC), polyvinyl alcohol (PVA), and β-cyclodextrin (βCD), through a simple and benign crosslinking strategy. Notably, the gel had sustained-release property in a wide temperature range of 4–35 ℃ and released ClO2 gas effectively for more than 30 days. Furthermore, a loss factor was proposed based on the incomplete release of the drug in the sustained release process to a chieve a good fit with the gas diffusion process. A new diffusion model was designed based on the Korsmeyer–Peppas model, and an excellent fit was obtained. This sustained-ClO2-gas-release gel provides theoretical and technical guidance for the development of sustained-disinfectant-release agents for use in space and offers new insights into the sustained release model of skeleton-soluble hydrogels.
Graphical abstract

Similar content being viewed by others
Introduction
The Covid-19 pandemic has made it essential to maintain public health safety(Yan et al. 2021; Han et al. 2022; Santos-Rosales et al. 2022). Currently, leading disinfectants used in the common environment include pyridine halide salts, ozone, ultraviolet lamps, hydrogen peroxide, and others(Wilson and Nayak 2019; Lowe et al. 2020; Liao et al. 2020; Bennet et al. 2021; Santos-Rosales et al. 2022). However, prolonged exposure to these disinfectants poses health risks(Preller et al. 1996; Park et al. 2017; Autier and Doré 2020; Samara et al. 2020; Zhang and Lu 2021). Thus, it is necessary to develop a disinfection device that is safe for humans and can function over extended periods of time.
Chlorine dioxide (ClO2) is the safest and most effective A1-level green disinfectant; it is widely used in many fields such as general disinfection, food preservation, medical care, and drinking water treatment(Sun et al. 2019; Chen et al. 2019; Chai et al. 2020; Lee et al. 2020). Owing to its high water solubility, ClO2 exhibits strong adsorption and penetration abilities towards microbial cell walls and cell membranes. Thus, is highly effective disinfective agent when it comes in direct contact with microorganisms. Further, ClO2 gas is a more effective ClO2-based disinfectant than the ClO2 solution because it has better diffusion ability, is easy to come in contact with microbial cells, and can react with cell materials to kill bacteria. (Du et al. 2002, 2003; Chai et al. 2020; Sadeghi et al. 2020). Currently, long-term controlled release is an important index for determining the practical applicability of ClO2 gas sustained-release devices(Ahmed et al. 2017; Liu et al. 2020). Therefore, polymer films, emulsions, hydrogels, aerogels, and inorganic porous materials are being tested for their potential use in ClO2 gas sustained-release devices(Palcsó et al. 2019). Such gas sustained-release devices are based on materials that can coat the ClO2 solution or adsorb ClO2 gas to achieve the sustained release of ClO2. The release time of most materials used in sustained-release devices iswithin 200 h, and this is attributed to the rapid detachment of ClO2 from the aqueous phase and the low content of ClO2. Thus, research on ClO2 sustained-release devices has now focused on increasing the load of ClO2 in materials and slowing down the release rate of ClO2 gas(Du et al. 2002; Chai et al. 2020; Sadeghi et al. 2020). The development of a sustained-release model is important for describing the process and laws of sustained-release of drugs. Among the current sustained-release models, only the zero-order, first-order, and Higuchi sustained-release models exhibit an excellent fitting for thesustained-release process of drugs with constant or decreasing sustained-release rate. Although the Korsmeyer–Peppas model can derive the diffusion law of narcotics and the dissolution laws of drug carriers, it fails to describe the loss of drugs in the sustained-release process; further, this model can not provide goodfitfor the sustained-release of medicines with incomplete release (Bhattacharyya et al. 2020; Saravanan et al. 2020; He et al. 2021).
A hydrogel is a polymer network system with a three-dimensional structure and water as dispersion medium. The excellent swelling performance and three-dimensional network structure comprising internal molecular chains make it suitable to transport drugs, and it is widely used in sustained drug release(Javanbakht et al. 2019). In this study, we used carboxymethyl cellulose (CMC), polyvinyl alcohol (PVA), and β-cyclodextrin (βCD) to prepare a ternary hydrogel (Gel 3). The sustained-release of ClO2 gas is shown in Fig. 1. A new drug sustained-release model (KP-Z) was designed based on the Korsmeyer–Peppas model. Unlike with the simple ClO2 solution, ClO2 gas can be produced and released via the chemical reaction between the chlorite and acid by attaching hydrogels loaded with sodium chlorite and citric acid. In the sustained-release process, the sodium chlorite and citric acid solution in hydrogels interact via interpenetration, and ClO2 is produced at the interface. The internal three-dimensional network structure of this gel slows down the release rate of the ClO2 gas; further, the adhesion and separation of the gel can afford the controlled release of ClO2 gas. This suggests that ClO2 gas has a wide range of potential applications, such as environmental disinfection, fruit and vegetable preservation.
Materials and methods
Experimental materials
CMC (2500–4500 mPa.s); PVA (Mw = 205,000); βCD (98%); and ECH were purchased from Aladdin Biochemical Technology Co., Ltd. Further, sodium hydroxide, sodium thiosulfate, potassium iodide, and potassium dichromate were obtained from the Tianjin Damao Chemical Reagent Factory). The materials were used as obtained without further purification.
Preparation of gel 2
The CMC, PVA, and ECH of the different qualities were added into deionized water containing 0.8 g/mL NaOH. The raw materials were stirred evenly, after which they were fully cross-linked by standing at room temperature for 5 h.
Preparation of gel 3
Gel 3 was prepared by dissolving 0.52 g CMC, 0.2 g PVA, and 1.6 g NaOH in 20 mL deionized water, followed by the addition of 0.16 g, 0.24 g, 0.32 g, 0.40 g, and 0.48 g βCD. Then, 1.2 mL epichlorohydrin was added after repeated stirring, and the solution was allowed to stand at room temperature for 5 h to from cross-links and obtain the product.
Material characterization
The infrared spectrum was measured using Fourier infrared spectrometer (TENSOR II, BRUKER Company, Germany) at room temperature from 4000–400 cm−1. The desktop scanning electron microscope (F16502, PHENOM, the Netherlands) obtained SEM images at an accelerated voltage of 5 kV. The universal material testing machine (3367, Enstrom Company, USA) was used to measure the compressive strength and the maximum deformation of the material. The X-ray photoelectron spectroscopy (ESCALAB 250XI + , Thermo Fisher Scientific, USA) obtained XPS images, and the hydrogel map was recorded using a Nikon D750 digital camera.
Swelling property measurement
The gel film with NaOH was removed and dried at 50 °C to constant weight. 0.2 g of the hydrogel film was weighed accurately, and the weight W0 was recorded. Next, the film is soaked in ultrapure water for some time until the weight becomes constant; this is considered as reaching the swelling equilibrium. Further, the swollen hydrogel is removed from the ultrapure water at every 100 min, the excess water on the surface of hydrogel is absorbed using absorbent paper, and the film is then weighed using an analytical balance; this weight is recorded as Wt. Each sample was tested in parallel three times and averaged. The swelling formula is given as.
where W0, Wt, and S represent the weight (g) of the gel before swelling, weight (g) of the gel after swelling, and swelling degree (g/g), respectively.
Drug loading and the release experiment of materials
The gels are soaked in a citric acid and sodium chlorite solution; once swelling balance is reached, 50 g of citric acid and sodium chlorite gels are cut using a circular knife die with an inner diameter of 44.4 mm. The cut gels are then placed in in a 100 mL beaker with an inner diameter of 44.4 mm, with the citric acid-containing gels in the upper layer and the sodium chlorite-containing gels in the lower layer. The cups filled with the gel are tied with PE wires and suspended in a 1 L wide-mouth reagent bottle, as indicated in Fig. 4d; three parallel samples are placed in each group.
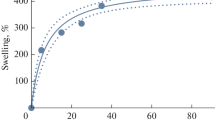
Characterization and swelling property of Gel 3. a FTIR spectra of starting materials and gel, b–g Gel microstructure in physical scale of 80 μm with different amount of βCD additions: 0, 0.16, 0.25, 0.32, 0.40, and 0.48 g, h Swelling ratios of Gel 3 and i Compressive stress and strains for Gels 2 and 3
Determination of ClO2 Release
ClO2 gas was absorbed using 10 mL of 5% potassium iodide solution and 6 mL of 1 mol/L H2SO4 solution. Every 24 h, the absorption solution is taken out and titrated with the prepared sodium thiosulfate solution (c = 0.01147 mol/L); then, the amount of the ClO2 released every 24 h is calculated based on the amount of the sodium thiosulfate solution consumed. The calculation formula is
where m, V1, and V0 represent the mass (mg) of ClO2, volume (mL) of sodium thiosulfate consumed by the sample, and volume (mL) of sodium thiosulfate consumed by the blank sample, respectively.
Measurement of effective ClO2 release rate
The ratio of the total effective release P of the sample to the initial effective ClO2 in the sodium chlorite of each concentration is calculated using,
where X, M, and Yn represent total effective release rate (%),daily release amount (mg) of the device, and total effective chlorine dioxide content (mg) contained in the gel in the theoretical calculation, respectively.
Fitting a sustained-release model
Fitting data were obtained from the actual release amount and release time of the sustained-release device, and model fitting was completed using IBM SPSS Statistics 23. The equations of the Higuchi, Korsmeyer–Peppas and KP-Z models are as follows:
Higuchi model:
Korsmeyer–Peppas model:
KP-Z model:
where Mt, M∞, k, n, t, and Qrepresent the accumulated release of ClO2 gas at time t, total theoretical release of ClO2 gas, reaction kinetic constant, diffusion index, release time of cumulative release rate of the drug, respectively. Further, in the KP-Z model, t represents the diffusion time (h), n1 represents diffusion index, and n2 represents loss factor.
Results and discussion
Synthesis and characterization of ternary hydrogel
The CMC, PVA, and βCD of different qualities were dissolved in the NaOH solution, and the aqueous solution was covalently cross-linked with epichlorohydrin (ECH) as the cross-linking agent to form Gel 3. The Fourier transform infrared (FTIR) spectra of starting materials and cross-linked products are illustrated in Fig. 2a. The intensity of − OH stretching vibration peak of the hydrogel at 3200–3600 cm−1 decreased for the CMC-PVA hydrogel (Gel 2) and Gel 3 because hydroxyl groups on the startingmaterials participated in cross-linking reaction(Alam et al. 2019). This confirm the successful cross-linking of the starting materials to form hydrogel. Further, few hydroxyl groups reduced the hydrogen bonds in the dried gel and improved the swelling property of the hydrogel. In the experiment with different βCD contents, the swelling ratio of Gel 3 first increased and then decreased with an increase in βCD content. Further, the highest swelling ratio of 2040.62 g/g was achieved when the βCD content was 0.24 g (13%). This swelling ratio is higher than that of most reported hydrogels, and this ultrahigh swelling ratio allows the gels to carry more drugs and provide longer sustained release times.
Sustained-release performance of Gel 3 under different conditions (a), (a1, Release process of ClO2 and effective release rate (b), (b1, at different temperatures) the release process and effective release rate of ClO2 under different reactant concentrations (c), (c1, the release process and effective release rate of ClO2 under different citric acid concentrations)
The molding performance of the gel is improved after adding βCD, as indicated in Fig. 2b–g. Further, the ring βCD becomes the rigid connection point of the three-dimensional network structure of the gel owing to itshigh crystallinity resulting in the formation of honeycomb microstructure inside the gel. This significantly improves the mechanical properties of the hydrogel and overcomes the issue of fragility of the CMC-PVA gel after reaching the swelling equilibrium. A comparison between Gel 2 and Gel 3 with the highest swelling rate indicated that Gel 3 had a compressive strength of 7.9 kPa after reaching the swelling equilibrium, and it was 220% higher than that before addition (Fig. 2h–i) and Fig. S3). Further, the need to increase the CMC content for achieving the same mechanical properties, affected the final swelling performance of the gel (Fig. S1). Further, the ring structure of βCD and a large number of hydroxyl groups on the surface as observed in the SEM images shown in Fig. 2b–g, provided greater cross-linking density at the connection point. The gel section observed under in SEM achieved a more regular mesh structure with an increase in the βCD content.
The optimum swelling rate of Gel 3 was lower than that of Gel 2 because of the decrease in the CMC and PVA content, which constituted the gel network (Fig. 1e and Fig. S3)(Nozaki et al. 1997; Tan et al. 2014; Sikder et al. 2019; Zhou et al. 2019; Kundu et al. 2019). However, the addition of βCD did not affect the swelling performance of the gel with the same content of CMC and PVA. Further, the blocking effect of cyclodextrin decreased the swelling rate of the gel, which effectively reduced the mutual penetration rate of sodium chlorite and citric acid at the contact interface. Gel 3 can achieve a sustained-release effect by decreasing the reaction rate and inhibiting the release of ClO2 gas.
Sustained-release performance of the gel for the release of ClO2 gas
50 g samples (Fig. 4d) were sealed in a 1 L wide-mouth sample bottle to evaluate the sustained-release performance of gel. The sustained-release performance of Gel 3 was tested under different ambient temperatures and different concentrations of reactants and citric acid. Owing to the formation of ClO2, the color of the gel gradually changed from colorless (translucent) to yellowish brown during the sustained-release process (Fig. S-7). The ambient temperature of the experimental groups with different concentrations of reactants and citric acid was set at 35 °C to speed up the experimental process.
Each gel device showed excellent sustained-release property even under the environmental conditions that exceeded room temperature (Fig. 3a). The actual sustained-release gel test device can prevent strawberries from spoilage for 18 days (see Fig. S-8). As shown in Fig. 3a, this gel effectively releases ClO2 gas for more than 40 days when the experimental temperature drops below 15 °C. Further, Fig. 3a1 shows the change in the total effective release rate of ClO2 at the same concentration and under different temperatures. The total effective release rate increased first and then decreased with an increase in the temperature. Figure 3b shows that the release rate of solid ClO2 at all concentrations increased first and then decreased, whereas the release amount at the initial stage of the reaction increased gradually. This is primarily because only one contact surface of sodium chlorite gel and citric acid gel can generate ClO2 at the initial stage; therefore, the amount of ClO2 generated in this process is alsosmall, and the diffusion driving force is small. Only a small amount of ClO2 can be released into the air through the layer network structure of the gel. With increase in time, the interaction between the sodium chlorite gel and citric acid gradually increases, and a large amount of ClO2 is generated. At this time, as driving force for diffusion gradually increase, and the release of ClO2 begins to increase. When the interaction between sodium chlorite gel and citric acid interrupts, the release of ClO2 reaches the highest point. As time progresses, ClO2 release by the gel gradually decreases because of the decrease in residual sodium chlorite and citric acid in the gel. Interestingly, Fig. 3c shows that increasing the citric acid concentration has negligible effect on the release process of ClO2. This phenomenon can be attributed to the decrease in the gel pH caused by the increase in the citric acid concentration. The driving force for diffusion in the process of sustained release decreases and the solubility of ClO2 in the gel increases. The total effective release rate increased slightly with an increase in the citric acid concentration becauseof the increase in the reaction rate. However, ClO2 was more soluble in the gel, and thus, it had no significant effect on the total effective release rate. In contrast,as shown in Fig. 3b1, higher reactant concentrations and lower release temperatures improve the overall effective release rate of ClO2 in devices.
Fitting of ClO2 release process
The Korsmeyer–Peppas and Higuchi models provide good fit for the ClO2 release under different conditions (Fig. 5). However, linear fitting may not accurately reflect the release pattern for incomplete release or skeleton corrosion. The Korsmeyer–Peppas model shows that a larger value of n corresponds to a release fraction that is further from 100% at the end of sustained release, resulting in a poorfit. Thus, the Korsmeyer–Peppas model is developed by introducing the “loss factor n2” to compensate for the incomplete release of the drugs. In addition to the accompanying skeleton-corrosion phenomenon, the KP-Z model that considers the existence of the skeleton corrosion in the sustained-release process is established.
The Higuchi, Korsmeyer–Peppas and KP-Z models were used to fit the gas release process under various experimental conditions. The diffusion factors N obtained by linear fitting under different temperatures, reactant concentrations, and citric acid concentrations were all greater than 0.890 when fitted with Korsmeyer–Peppas model (Fig. 5), indicating that this is a typical non-Fick diffusion process with skeleton corrosion. The fitting results of the Higuchi and Korsmeyer–Peppas models often deviate from the actual release results because of the low release flux in the early and late stages of the reaction during linear fitting. The nonlinear fitting of the KP-Z model provides a better fitting effect. Further, the value of n2 indirectly reflects the total effective release rate of gel; a larger loss factor corresponds to a smaller effective release rate.
Conclusion
We developed a ClO2 gas sustained-release device based on a high-swelling ternary hydrogel and explained the sustained-release process by developing a KP-Z sustained-release model. On the one hand, the inherent network structure in the hydrogel slowed down the reaction between sodium chlorite and citric acid and reduced the diffusion rate of the ClO2 generated at the interface to the outside. This significantly increased the ClO2 gas sustained-release performance of the material, allowing the device to sustain the release for more than 30 days. further, the strawberries stored in a refrigerated environment with the device did not deteriorate within 18 (see S-8). In addtion, the separable structure made the release process of the ClO2 gas controllable.
This paper presented a ClO2 sustained-release device, with excellent sustained-release performance, based on a simple and benign crosslinking reaction for environmental disinfection. Owing to the unique sustained-release properties, the developed hydrogel based sustained-release device is expected to replace some existing environmental disinfectants and inspire the design of the internal structures in sustained drug-release devices.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Ahmed ST, Bostami ABMR, Mun H-S, Yang C-J (2017) Efficacy of chlorine dioxide gas in reducing Escherichia coli and Salmonella from broiler house environments. J Appl Poul Res 26:84–88. https://doi.org/10.3382/japr/pfw048
Alam MN, Islam MdS, Christopher LP (2019) Sustainable production of cellulose-based hydrogels with superb absorbing potential in physiological saline. ACS Omega 4:9419–9426. https://doi.org/10.1021/acsomega.9b00651
Autier P, Doré J-F (2020) Ultraviolet radiation and cutaneous melanoma: a historical perspective. Melanoma Res 30:113–125. https://doi.org/10.1097/CMR.0000000000000609
Bennet D, Harris AF, Lacombe J et al (2021) Evaluation of supercritical CO2 sterilization efficacy for sanitizing personal protective equipment from the coronavirus SARS-CoV-2. Sci Total Environ 780:146519. https://doi.org/10.1016/j.scitotenv.2021.146519
Bhattacharyya SK, Dule M, Paul R et al (2020) Carbon dot cross-linked gelatin nanocomposite hydrogel for pH-sensing and pH-responsive drug delivery. ACS Biomater Sci Eng 6:5662–5674. https://doi.org/10.1021/acsbiomaterials.0c00982
Chai H-E, Hwang C-A, Huang L et al (2020) Feasibility and efficacy of using gaseous chlorine dioxide generated by sodium chlorite-acid reaction for decontamination of foodborne pathogens on produce. Food Control 108:106839. https://doi.org/10.1016/j.foodcont.2019.106839
Chen X-Q, Pang G-X, Shen W-H et al (2019) Preparation and characterization of the ribbon-like cellulose nanocrystals by the cellulase enzymolysis of cotton pulp fibers. Carbohyd Polym 207:713–719. https://doi.org/10.1016/j.carbpol.2018.12.042
Du J, Han Y, Linton RH (2003) Efficacy of chlorine dioxide gas in reducing Escherichia coli O157:H7 on apple surfaces. Food Microbiol 20:583–591. https://doi.org/10.1016/S0740-0020(02)00129-6
Du J, Han Y, Linton RH (2002) Inactivation by chlorine dioxide gas (ClO2) of Listeria monocytogenes spotted onto different apple surfaces. Food Microbiol 19:481–490. https://doi.org/10.1006/fmic.2002.0501
Han S, Kim J, Lee Y et al (2022) Transparent air filters with active thermal sterilization. Nano Lett 22:524–532. https://doi.org/10.1021/acs.nanolett.1c02737
He X, Yuan Z, Gaeke S et al (2021) Laser-activated drug implant for controlled release to the posterior segment of the eye. ACS Appl Bio Mater 4:1461–1469. https://doi.org/10.1021/acsabm.0c01334
Javanbakht S, Pooresmaeil M, Namazi H (2019) Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohyd Polym 208:294–301. https://doi.org/10.1016/j.carbpol.2018.12.066
Kundu D, Mondal SK, Banerjee T (2019) Development of β-cyclodextrin-cellulose/hemicellulose-based hydrogels for the removal of Cd(II) and Ni(II): synthesis, kinetics, and adsorption aspects. J Chem Eng Data 64:2601–2617. https://doi.org/10.1021/acs.jced.9b00088
Lee H, Ryu J-H, Kim H (2020) Antimicrobial activity of gaseous chlorine dioxide against Aspergillus flavus on green coffee beans. Food Microbiol 86:103308. https://doi.org/10.1016/j.fm.2019.103308
Liao L, Xiao W, Zhao M et al (2020) Can N95 respirators be reused after disinfection? How many times? ACS Nano 14:6348–6356. https://doi.org/10.1021/acsnano.0c03597
Liu X, Jiao W, Du Y et al (2020) Chlorine dioxide controls green mold caused by penicillium digitatum in citrus fruits and the mechanism involved. J Agric Food Chem 68:13897–13905. https://doi.org/10.1021/acs.jafc.0c05288
Lowe JJ, Paladino KD, Farke JD, et al (2020) N95 filtering facepiece respirator ultraviolet germicidal irradiation (UVGI) process for decontamination and reuse. University of Nebraska Medical Center
Nozaki T, Maeda Y, Kitano H (1997) Cyclodextrin gels which have a temperature responsiveness. J Polym Sci, Part a: Polym Chem 35:1535–1541. https://doi.org/10.1002/(SICI)1099-0518(199706)35:8%3c1535::AID-POLA22%3e3.0.CO;2-7
Palcsó B, Moldován Z, Süvegh K et al (2019) Chlorine dioxide-loaded poly(acrylic acid) gels for prolonged antimicrobial effect. Mater Sci Eng, C 98:782–788. https://doi.org/10.1016/j.msec.2019.01.043
Park D-U, Ryu S-H, Lim H-K et al (2017) Types of household humidifier disinfectant and associated risk of lung injury (HDLI) in South Korea. Sci Total Environ 596–597:53–60. https://doi.org/10.1016/j.scitotenv.2017.04.040
Preller L, Doekes G, Heederik D et al (1996) Disinfectant use as a risk factor for atopic sensitization and symptoms consistent with asthma: an epidemiological study. Eur Respir J 9:1407–1413. https://doi.org/10.1183/09031936.96.09071407
Sadeghi K, Kasi G, Ketsuk P et al (2020) A polymeric chlorine dioxide self-releasing sheet to prolong postharvest life of cherry tomatoes. Postharvest Biol Technol 161:111040. https://doi.org/10.1016/j.postharvbio.2019.111040
Samara F, Badran R, Dalibalta S (2020) Are disinfectants for the prevention and control of COVID-19 SAFE? Health Secur 18:496–498. https://doi.org/10.1089/hs.2020.0104
Santos-Rosales V, López-Iglesias C, Sampedro-Viana A et al (2022) Supercritical CO2 sterilization: an effective treatment to reprocess FFP3 face masks and to reduce waste during COVID-19 pandemic. Sci Total Environ 826:154089. https://doi.org/10.1016/j.scitotenv.2022.154089
Saravanan A, Maruthapandi M, Das P et al (2020) Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl Bio Mater 3:8023–8031. https://doi.org/10.1021/acsabm.0c01104
Sikder MdT, Rahman MdM, Jakariya Md et al (2019) Remediation of water pollution with native cyclodextrins and modified cyclodextrins: a comparative overview and perspectives. Chem Eng J 355:920–941. https://doi.org/10.1016/j.cej.2018.08.218
Sun X, Baldwin E, Bai J (2019) Applications of gaseous chlorine dioxide on postharvest handling and storage of fruits and vegetables—a review. Food Control 95:18–26. https://doi.org/10.1016/j.foodcont.2018.07.044
Tan S, Ladewig K, Fu Q et al (2014) Cyclodextrin-based supramolecular assemblies and hydrogels: recent advances and future perspectives. Macromol Rapid Commun 35:1166–1184. https://doi.org/10.1002/marc.201400080
Wilson AJ, Nayak S (2019) Disinfection, sterilization and disposables. Anaesth Intensive Care Med 20:603–608. https://doi.org/10.1016/j.mpaic.2019.09.013
Yan H, Hu B, Wang R (2021) Air-source heat pump heating based water vapor compression for localized steam sterilization applications during the COVID-19 pandemic. Renew Sustain Energy Rev 145:111026. https://doi.org/10.1016/j.rser.2021.111026
Zhang C, Lu J (2021) Optimizing disinfectant residual dosage in engineered water systems to minimize the overall health risks of opportunistic pathogens and disinfection by-products. Sci Total Environ 770:145356. https://doi.org/10.1016/j.scitotenv.2021.145356
Zhou L, Xu Z, Yi K et al (2019) Efficient remediation of 2,4-dichlorophenol from aqueous solution using β-cyclodextrin-based submicron polymeric particles. Chem Eng J 360:531–541. https://doi.org/10.1016/j.cej.2018.11.196
Acknowledgments
We thank the Guangxi Key Laboratory of Clean Pulp&Papermaking and pollution Control, College of Light Industry and Food Engineering, Guangxi University, Nanning 530004, China and South China University of Technology, Guangzhou 510000, China.
Funding
This work was supported by the Guangxi Natural Science Foundation [No. GXNSFAA297042]; Guangxi Bossco Environmental Protection Technology (Bossco) [No.AA17129006]; Guangxi Key Laboratory of Clean Pulp & Papermaking and Pollution Control [No. 2021KF53]; and Guangdong Basic and Applied Basic Research Foundation [No. 2021A1515010899]. Guangdong Natural Science Foundation [No. 2022A1515011416]; Shantou Science and Technology Plan Project [No. 210719155863891].
Author information
Authors and Affiliations
Contributions
HZ and YW initiated and designed the research. YW and HZ wrote performed research. HZ and YW wrote the manuscript. HZ, YW, LH, GC, ZW and QM curated data. HZ, LH and GC, interpreted and annotated the data. HZ, LH, GC, ZW, YW, QM, YL, XW, CH. and QC analyzed the data and results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent for publication
All authors agree to publish in cellulose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Zhao, H., Huang, L. et al. Development of chlorine dioxide sustained-release device using carboxymethyl cellulose-polyvinyl alcohol-β-cyclodextrin ternary hydrogel and a new sustained-release kinetic model. Cellulose 30, 3073–3082 (2023). https://doi.org/10.1007/s10570-023-05070-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05070-6