Abstract
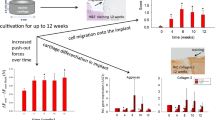
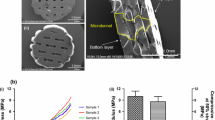
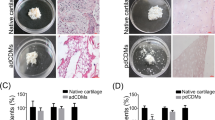
Bacterial nanocellulose (BNC) shows high biocompatibility as wound dressing or dura mater, blood vessel, and cartilage implant. Three-dimensional perforation (3-D-∅) favors migration of chondrocytes into the BNC and cartilage matrix formation. Thus, the regenerative capacity of 3-D-∅ BNC implants was tested in a standardized bovine cartilage punch model. Cartilage rings containing a central defect with an outer diameter of 6 mm and an inner diameter of 2 mm were prepared from the trochlear groove (femur-patellar articulation site). Three-D-∅ BNC implants (cell-free or cell-loaded) were cultured inside the cartilage rings for up to 12 weeks. Cartilage-BNC-constructs were then investigated by histology (hematoxylin/eosin; safranin O) and immunohistology (aggrecan, collagens 1 and 2), as well as for protein content, RNA expression, and implant push-out force. Cartilage-BNC-constructs remained vital with preserved matrix integrity during culture and almost no loss of matrix-bound proteoglycan (aggrecan) or collagen 2 from ‘host’ cartilage (with very limited quantities of collagen 1). Interestingly, 3-D-∅ BNC implants displayed: (1) significantly increased superficial, but also 3-D cell seeding over time (cell-loaded significantly earlier than cell-free); (2) progressively increased aggrecan/collagen 1 and collagen 2/collagen 1 mRNA ratios, as well as aggrecan and collagen 2 protein levels; and (3) significantly increased push-out forces over time (cell-loaded). Progressively increasing cell seeding and chondrogenic differentiation suggest beginning cartilage regeneration of the 3-D-∅ BNC in this model system, and indicate an excellent potential of 3-D-∅ BNC as a cartilage replacement material. Cell-loading may favor implant performance by accelerating cell colonization.
Graphical abstract












Similar content being viewed by others
References
Ahrem H, Pretzel D, Endres M, Conrad D, Courseau J, Muller H, Jaeger R, Kaps C, Klemm DO, Kinne RW (2014) Laser-structured bacterial nanocellulose hydrogels support ingrowth and differentiation of chondrocytes and show potential as cartilage implants. Acta Biomater 10:1341–1353
Apelgren P, Amoroso M, Lindahl A, Brantsing C, Rotter N, Gatenholm P, Kolby L (2017) Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS ONE 12:e0189428
Avila HM, Schwarz S, Feldmann EM, Mantas A, von Bomhard A, Gatenholm P, Rotter N (2014) Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl Microbiol Biotechnol 98:7423–7435
Avila HM, Feldmann EM, Pleumeekers MM, Nimeskern L, Kuo W, de Jong WC, Schwarz S, Muller R, Hendriks J, Rotter N, van Osch GJ, Stok KS, Gatenholm P (2015) Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials 44:122–133
Bachmann G, Basad E, Lommel D, Steinmeyer J (2004) MRI in the follow-up of matrix-supported autologous chondrocyte transplantation (MACI) and microfracture. Radiologe 44:773–782
Bartlett W, Krishnan SP, Skinner JA, Carrington RWJ, Briggs TWR, Bentley G (2006) Collagen-covered versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a comparison of tourniquet times. Eur J Orthop Surg Traumatol 16:315–317
Bodin A, Concaro S, Brittberg M, Gatenholm P (2007) Bacterial cellulose as a potential meniscus implant. J Tissue Eng Regen Med 1:406–408
Chandrasekhar S, Esterman MA, Hoffman HA (1987) Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Anal Biochem 161:103–108
Dell’Accio F, De Bari C, El Tawil NM, Barone F, Mitsiadis TA, O’Dowd J, Pitzalis C (2006) Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res Ther 8:R139
Dewan AK, Gibson MA, Elisseeff JH, Trice ME (2014) Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res Int 2014:272481
Dumanli AG (2017) Nanocellulose and its composites for biomedical applications. Curr Med Chem 24:512–528
Dunzel A, Rudiger T, Pretzel D, Kopsch V, Endres M, Kaps C, Fohr P, Burgkart RH, Linss S, Kinne RW (2013) The bovine cartilage punch model: a tool for the in vitro analysis of biomaterials and cartilage regeneration. Orthopade 42:254–261
Erggelet C, Endres M, Neumann K, Morawietz L, Ringe J, Haberstroh K, Sittinger M, Kaps C (2009) Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J Orthop Res 27:1353–1360
Everest A, Filip J, Safarikova M, Salih V, Keshavarz T, Knowles J, Roy I (2016) Composite scaffolds for cartilage tissue engineering based on natural polymers of bacterial origin, thermoplastic poly(3-hydroxybutyrate) and micro-fibrillated bacterial cellulose. Polym Int 65:780–791
Farndale RW, Buttle DJ, Barrett AJ (1986) Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883:173–177
Feldmann EM, Sundberg JF, Bobbili B, Schwarz S, Gatenholm P, Rotter N (2013) Description of a novel approach to engineer cartilage with porous bacterial nanocellulose for reconstruction of a human auricle. J Biomater Appl 28:626–640
Gatenholm P (2017) Cellulose nanofibrillar bioink for 3D bioprinting for cell culturing, tissue engineering and regenerative medicine applications. In: United States Patent Application Publication, ed. U. S. P. Office. United States: Celllink AB
Gavenis K, Schneider U, Maus U, Mumme T, Muller-Rath R, Schmidt-Rohlfing B, Andereya S (2012) Cell-free repair of small cartilage defects in the Goettinger minipig: which defect size is possible? Knee Surg Sports Traumatol Arthrosc 20:2307–2314
Gavenis K, Heussen N, Hofman M, Andereya S, Schneider U, Schmidt-Rohlfing B (2014) Cell-free repair of small cartilage defects in the Goettinger minipig: the effects of BMP-7 continuously released by poly(lactic-co-glycolid acid) microspheres. J Biomater Appl 28:1008–1015
Gillogly SD, Wheeler KS (2015) Autologous chondrocyte implantation with collagen membrane. Sports Med Arthrosc 23:118–124
Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N (2015) Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage 6:82–97
Horbert V, Xin L, Foehr P, Brinkmann O, Bungartz M, Burgkart R. H, Graeve T, Kinne RW (2018) In vitro analysis of cartilage regeneration using a collagen Type I hydrogel (CaReS) in the Bovine Cartilage Punch Model. Cartilage, 1947603518756985
Horbert V, Foehr P, Kramer F, Udhardt U, Bungartz M, Brinkmann O, Burgkart RH, Klemm DO, Kinne RW (2019) In vitro analysis of the potential cartilage implant bacterial nanocellulose using the bovine cartilage punch model. Cellulose 26. https://doi.org/10.1007/s10570-019-02260-z (This issue)
Hunter CJ, Levenston ME (2004) Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng 10:736–746
Hunziker EB (2002) Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 10:432–463
Klemm D, Schumann D, Udhardt U, Marsch S (2001) Bacterial synthesized cellulose—artificial blood vessels for microsurgery. Prog Polym Sci 26:1561–1603
Kon E, Verdonk P, Condello V, Delcogliano M, Dhollander A, Filardo G, Pignotti E, Marcacci M (2009) Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med 37(Suppl 1):156S–166S
Kowalska-Ludwicka K, Cala J, Grobelski B, Sygut D, Jesionek-Kupnicka D, Kolodziejczyk M, Bielecki S, Pasieka Z (2013) Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch Med Sci 9:527–534
Kumbhar JV, Jadhav SH, Bodas DS, Barhanpurkar-Naik A, Wani MR, Paknikar KM, Rajwade JM (2017) In vitro and in vivo studies of a novel bacterial cellulose-based acellular bilayer nanocomposite scaffold for the repair of osteochondral defects. Int J Nanomed 12:6437–6459
Lang N, Merkel E, Fuchs F, Schumann D, Klemm D, Kramer F, Mayer-Wagner S, Schroeder C, Freudenthal F, Netz H, Kozlik-Feldmann R, Sigler M (2015) Bacterial nanocellulose as a new patch material for closure of ventricular septal defects in a pig model. Eur J Cardiothorac Surg 47:1013–1021
Lotz MK, Otsuki S, Grogan SP, Sah R, Terkeltaub R, D’Lima D (2010) Cartilage cell clusters. Arthritis Rheum 62:2206–2218
Luo H, Gu F, Xiong G, Hu D, Zhu Y, Wan Y (2016) A multichanneled bacterial cellulose scaffold for 3 D in vitro cancer culture. Cellul Chem Technol 50:49–56
Markstedt K, Mantas A, Tournier I, Martinez Avila H, Hagg D, Gatenholm P (2015) 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 16:1489–1496
Moller T, Amoroso M, Hagg D, Brantsing C, Rotter N, Apelgren P, Lindahl A, Kolby L, Gatenholm P (2017) In vivo chondrogenesis in 3D bioprinted human cell-laden hydrogel constructs. Plast Reconstr Surg Glob Open 5:e1227
Morales TI (2007) Chondrocyte moves: clever strategies? Osteoarthritis Cartil 15:861–871
Moretti M, Wendt D, Schaefer D, Jakob M, Hunziker EB, Heberer M, Martin I (2005) Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J Biomech 38:1846–1854
Moritz S, Wiegand C, Wesarg F, Hessler N, Muller FA, Kralisch D, Hipler UC, Fischer D (2014) Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int J Pharm 471:45–55
Muller M, Ozturk E, Arlov O, Gatenholm P, Zenobi-Wong M (2017) Alginate sulfate-nanocellulose bioinks for cartilage bioprinting applications. Ann Biomed Eng 45:210–223
Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, Loreto C, Castorina S (2014) New perspectives for articular cartilage repair treatment through tissue engineering: a contemporary review. World J Orthop 5:80–88
Napavichayanun S, Yamdech R, Aramwit P (2016) The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: in vitro, in vivo and clinical studies. Arch Dermatol Res 308:123–132
Nguyen D, Hagg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, Kalogeropoulos T, Zaunz S, Concaro S, Brittberg M, Lindahl A, Gatenholm P, Enejder A, Simonsson S (2017) Cartilage tissue engineering by the 3D bioprinting of iPS Cells in a nanocellulose/alginate bioink. Sci Rep 7:658
Nimeskern L, Martinez Avila H, Sundberg J, Gatenholm P, Muller R, Stok KS (2013) Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J Mech Behav Biomed Mater 22:12–21
Picheth GF, Pirich CL, Sierakowski MR, Woehl MA, Sakakibara CN, de Souza CF, Martin AA, da Silva R, de Freitas RA (2017) Bacterial cellulose in biomedical applications: a review. Int J Biol Macromol 104:97–106
Pretzel D, Linss S, Ahrem H, Endres M, Kaps C, Klemm D, Kinne RW (2013) A novel in vitro bovine cartilage punch model for assessing the regeneration of focal cartilage defects with biocompatible bacterial nanocellulose. Arthritis Res Ther 15:R59
Saska S, Teixeira LN, de Castro Raucci LMS, Scarel-Caminaga RM, Franchi LP, Dos Santos RA, Santagneli SH, Capela MV, de Oliveira PT, Takahashi CS, Gaspar AMM, Messaddeq Y, Ribeiro SJL, Marchetto R (2017) Nanocellulose-collagen-apatite composite associated with osteogenic growth peptide for bone regeneration. Int J Biol Macromol 103:467–476
Schramm M, Hestrin S (1954) Factors affecting production of cellulose at the air/liquid interface of a culture of Acetobacter xylinum. J Gen Microbiol 11:123–129
Schumann DA, Wippermann J, Klemm DO, Kramer F, Koth D, Kosmehl H, Wahlers T, Salehi-Gelani S (2009) Artificial vascular implants from bacterial cellulose: preliminary results of small arterial substitutes. Cellulose 16:877–885
Steinwachs M, Kreuz PC (2007) Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy 23:381–387
Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan DL, Brittberg M, Gatenholm P (2005) Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 26:419–431
Tayeb AH, Amini E, Ghasemi S, Tajvidi M (2018) Cellulose nanomaterials-binding properties and applications: a review. Molecules 23:2684
Theodoropoulos JS, De Croos JN, Park SS, Pilliar R, Kandel RA (2011) Integration of tissue-engineered cartilage with host cartilage: an in vitro model. Clin Orthop Relat Res 469:2785–2795
Ullah H, Wahid F, Santos HA, Khan T (2016) Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr Polym 150:330–352
Vinardell T, Thorpe SD, Buckley CT, Kelly DJ (2009) Chondrogenesis and integration of mesenchymal stem cells within an in vitro cartilage defect repair model. Ann Biomed Eng 37:2556–2565
Wang Y, Yuan X, Yu K, Meng H, Zheng Y, Peng J, Lu S, Liu X, Xie Y, Qiao K (2018) Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials 171:118–132
Wippermann J, Schumann D, Klemm D, Kosmehl H, Salehi-Gelani S, Wahlers T (2009) Preliminary results of small arterial substitute performed with a new cylindrical biomaterial composed of bacterial cellulose. Eur J Vasc Endovasc Surg 37:592–596
Xiong G, Luo H, Zhang C, Zhu Y, Wan Y (2015) Enhanced biological behavior of bacterial cellulose scaffold by creation of macropores and surface immobilization of collagen. Macromol Res 23:734–740
Ye K, Di Bella C, Myers DE, Choong PF (2014) The osteochondral dilemma: review of current management and future trends. ANZ J Surg 84:211–217
Yin N, Stilwell MD, Santos TMA, Wang H, Weibel DB (2015) Agarose particle-templated porous bacterial cellulose and its application in cartilage growth in vitro. Acta Biomater 12:129–138
Yin N, Chen SY, Cao YM, Wang HP, Wu QK (2017) Improvement in mechanical properties and biocompatibility of biosynthetic bacterial cellulose/lotus root starch composites. Chin J Poly Sci 35:354–364
Zhu X, Chen T, Feng B, Weng J, Duan K, Wang J, Lu X (2018) Biomimetic bacterial cellulose-enhanced double-network hydrogel with excellent mechanical properties applied for the osteochondral defect repair. ACS Biomater Sci Eng 4:3534–3544
Acknowledgments
The authors are grateful to Cordula Müller, Bärbel Ukena, Ulrike Körner, and Tobias Fiedler (Experimental Rheumatology Unit, Jena University Hospital) for expert technical assistance and qRT-PCR analyses, Maren Siedentop, Daniela Warnecke, and Fabian Holzner (Biomechanics Laboratory, Klinikum rechts der Isar, Technische Universität München) for expert biomechanical testing of implant push-out forces, Hannes Ahrem (Jenpolymer Materials Ltd & Co. KG, Jena, Germany) for producing the BNC hydrogels, and Daniel Conrad and Hartmut Müller (Günter-Köhler-Institut für Fügetechnik und Werkstoffprüfung GmbH, Jena) for laser structuring of the BNC hydrogels.
We gratefully acknowledge the partial financial support of the Bundesministerium für Bildung und Forschung (BMBF), Grant References 13N12601 and 0315577C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Horbert, V., Boettcher, J., Foehr, P. et al. Laser perforation and cell seeding improve bacterial nanocellulose as a potential cartilage implant in the in vitro cartilage punch model. Cellulose 26, 647–664 (2019). https://doi.org/10.1007/s10570-019-02286-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02286-3