Abstract
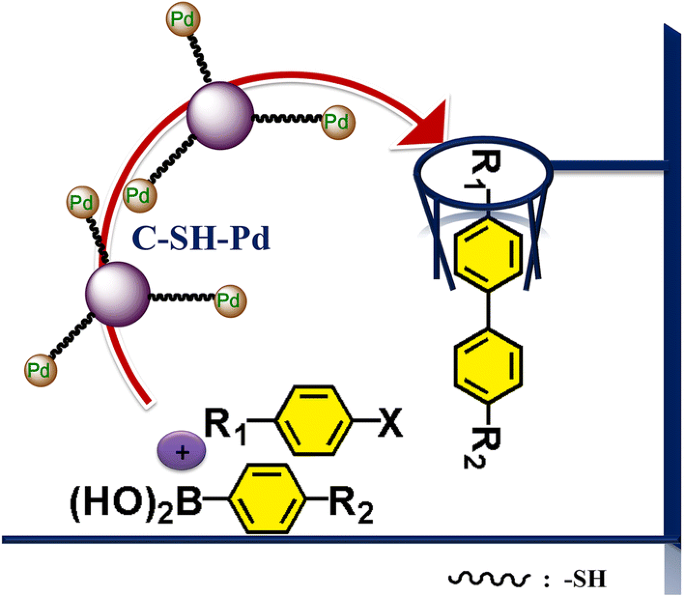
Novel thiol functionalized xylose hydrochar microspheres supported palladium nanoparticles (C–SH–Pd) were synthesized by gentle heating of palladium (II) acetate and thiol functionalized xylose hydrochar (C–SH) in ethanol. The as-prepared C–SH–Pd exhibited high catalytic activity towards Suzuki reactions with a yield of high up to 100%. Moreover, it could be reused for at least five times without heavily loss of the catalytic activity. The amount of palladium entrapped on C-SH microspheres was measured by AAS and found to be 1.42 mmol/g. Leaching studies showed that the filtrate contained less than 0.2 ppm Pd. Due to the superior catalytic performance and stability of the C–SH–Pd catalyst, it can be exploited in other cross coupling reactions in the long run.
Graphic Abstract








Similar content being viewed by others
References
Johansson Seechurn CC, Kitching MO, Colacot TJ, Snieckus V (2012) Angew Chem Int Ed Engl 51:5062–5085
Norio M, Akira S (1995) Chem Rev 95:2457–2483
Kunfi A, May Z, Németh P, London G (2018) J Catal 361:84–93
Xiao Q, Sarina S, Jaatinen E, Jia JF, Arnold DP, Liu HW, Zhu HY (2014) Green Chem 16:4272–4285
Puthiaraj P, Pitchumani K (2014) Green Chem 16:4223–4233
Yavuz K, Küçükbay H (2018) Appl Organomet Chem 32:e3897
Dong WH, Zhang L, Wang CH, Feng C, Shang NZ, Gao ST, Wang C (2016) RSC Adv 6:37118–37123
Rathi AK, Gawande MB, Pechousek J, Tucek J, Aparicio C, Petr M, Tomanec O, Krikavova R, Travnicek Z, Varma RS, Zboril R (2016) Green Chem 18:2363–2373
Veisi H, Manesh AA, Eivazi N, Faraji AR (2015) RSC Adv 5:20098–20107
Camp JE, Dunsford JJ, Dacosta OSG, Blundell RK, Adams J, Britton J, Smith RJ, Bousfield TW, Fay MW (2016) RSC Adv 6:16115–16121
Lichtenegger GJ, Maier M, Hackl M, Khinast JG, Gössler W, Griesser T, Phani Kumar VS, Gruber-Woelfler H, Deshpande PA (2017) J Mol Catal A 426:39–51
Gholinejad M, Najera C, Hamed F, Seyedhamzeh M, Bahrami M, Kompany-Zareh M (2017) Tetrahedron 73:5585–5592
Veisi H, Gholami J, Ueda H, Mohammadi P, Noroozi M (2015) J Mol Catal A 396:216–223
Gholinejad M, Zareh F, Nájera C (2018) Appl Organomet Chem 32:e3984
Huo JJ, Johnson RL, Duan P, Pham HN, Mendivelso-Perez D, Smith EA, Datye AK, Schmidt-Rohr K, Shanks BH (2018) Catal Sci Technol 8:1151–1160
Diyarbakir S, Can H, Metin O (2015) ACS Appl Mater Interfaces 7:3199–3206
Elazab HA, Siamaki AR, Moussa S, Gupton BF, El-Shall MS (2015) Appl Catal A 491:58–69
Jadhav S, Kumbhar A, Salunkhe R (2015) Appl Organomet Chem 29:339–345
Mori K, Masuda S, Tanaka H, Yoshizawa K, Che M, Yamashita H (2017) Chem Commun 53:4677–4680
Ayodele OB, Farouk HU, Mohammed J, Uemura Y, Daud WMAW (2015) J Mol Catal A 400:179–186
Fu WQ, Zhang L, Tang TD, Ke QP, Wang S, Hu JB, Fang GY, Li JX, Xiao FS (2011) J Am Chem Soc 133:15346–15349
Le XD, Dong ZP, Liu YS, Jin ZC, Huy TD, Le MD, Ma JT (2014) J Mater Chem A 2:19696–19706
Baran T, Menteş A (2016) J Mol Struct 1122:111–116
Kang SM, Li XL, Fan J, Chang J (2012) Ind Eng Chem Res 51:9023–9031
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S (2010) Nat Commun 1:56
Hitzl M, Mendez A, Owsianiak M, Renz M (2018) J Environ Chem Eng 6:7029–7034
James WL, Michelle K, Barbara RE, Sokwon P, Buchanan AC III, Charles TG, Robert CB (2010) Environ Sci Technol 44:7970–7974
Dumroese RK, Heiskanen J, Englund K, Tervahauta A (2011) Biomass Bioenergy 35:2018–2027
Wang LL, Wang XF, Zou B, Ma XY, Qu YN, Rong CG, Li Y, Su Y, Wang ZC (2011) Bioresour Technol 102:8220–8224
Liu ZG, Zhang FS (2011) Desalination 267:101–106
Zhu XD, Liu YC, Qian F, Zhou C, Zhang SC, Chen JM (2014) Bioresour Technol 154:209–214
Genovese M, Lian K (2017) J Mater Chem A 5:3939–3947
Liu WJ, Jiang H, Yu HQ (2015) Chem Rev 115:12251–12285
Tan XF, Liu SB, Liu YG, Gu YL, Zeng GM, Hu XJ, Wang X, Liu SH, Jiang LH (2017) Bioresour Technol 227:359–372
Wang XX, Hu PB, Xue FJ, Wei YP (2014) Carbohydr Polym 114:476–483
Baran NY, Baran T, Mentes A (2018) Carbohydr Polym 181:596–604
Frindy S, Primo A, Lahcini M, Bousmina M, Garcia H, Kadib AE (2015) Green Chem 17:1893–1898
Hajipour AR, Sadeghi AR, Khorsandi Z (2018) Appl Organomet Chem 32:e4112
Kardanpour R, Tangestaninejad S, Mirkhani V, Moghadam M, Mohammadpoor-Baltork I, Khosropour AR, Zadehahmadi F (2014) J Organomet Chem 761:127–133
Sevilla M, Fuertes AB (2009) Chemistry 15:4195–4203
Oliveira RL, He W, Gebbink RJMK, de Jong KP (2015) Catal Sci Technol 5:1919–1928
Sabounchei SJ, Ahmadi M, Nasri Z, Shams E, Panahimehr M (2013) Tetrahedron Lett 54:4656–4660
Crudden CM, Sateesh M, Lewis R (2005) J Am Chem Soc 127:10045–10050
Sobhi HR, Ghambarian M, Esrafili A, Behbahani M (2017) Microchim Acta 184:2317–2323
Yılmaz Ş, Şahan T, Karabakan A (2017) Korean J Chem Eng 34:2225–2235
Chen W, Zhong LX, Peng XW, Wang K, Chen ZF, Sun RC (2014) Catal Sci Technol 4:1426–1435
Evangelisti C, Panziera N, Pertici P, Vitulli G, Salvadori P, Battocchio C, Polzonetti G (2009) J Catal 262:287–293
Xie ZL, White RJ, Weber J, Taubert A, Titirici MM (2011) J Mater Chem 21:7434–7442
Chen W, Zhong LX, Peng XW, Lin JH, Sun RC (2014) Cellulose 21:125–137
Acknowledgements
We wish to thank for the National Natural Science Foundation of China (3197161, 31430092, 21736003), Guangdong Natural Science Funds for Distinguished Young Scholar (2016A030306027, 2017A030306029), Guangdong Natural Science Funds (2017A030313130), Guangzhou science and technology funds (201904010078), State Key Laboratory of Pulp and Paper Engineering and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, P., Zeng, X., Du, F. et al. Palladium Nanoparticles Anchored on Thiol Functionalized Xylose Hydrochar Microspheres: An Efficient Heterogeneous Catalyst for Suzuki Cross-Coupling Reactions. Catal Lett 150, 1011–1019 (2020). https://doi.org/10.1007/s10562-019-02984-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02984-4