Abstract
Neuropilins (NRPs) are transmembrane proteins involved in vascular and nervous system development by regulating angiogenesis and axon guidance cues. Several published reports have established their role in tumorigenesis. NRPs are detectable in tumor cells of several cancer types and participate in cancer progression. NRP2 is also expressed in endothelial and immune cells in the tumor microenvironment and promotes functions such as lymphangiogenesis and immune suppression important for cancer progression. In this review, we have taken a comprehensive approach to discussing various aspects of NRP2-signaling in cancer, including its regulation, functional significance in cancer progression, and how we could utilize our current knowledge to advance the studies and target NRP2 to develop effective cancer therapies.
Similar content being viewed by others
1 Introduction
Neuropilins (NRPs) are non-kinase single-pass transmembrane receptor proteins. The genetic sequences of neuropilins are conserved in the vertebrates [1]. They are actively involved in the neural crest migration during the development of vertebrates and participate in the regulation of axon guidance cues. Neuropilins are also involved in the development of blood and lymphatic endothelial cells [1, 2]. There are two prominent family members of NRPs, namely neuropilin-1 (NRP1) and neuropilin-2 (NRP2). Interestingly, the protein levels of NRP2, but not that of NRP1, are maintained during metabolic stress, nutrient starvation, and hypoxia that are typically associated with cancer progression and metastasis [3]. It therefore makes NRP2 a potential therapeutic target in cancer and presents opportunities for further research. Throughout this review, we have discussed our current knowledge of NRP2’s functions in the development and progression of cancer and how it can be therapeutically targeted. For the detailed information regarding NRPs in general, the readers can refer to some other review articles [4,5,6]. During cancer progression, NRP2 function is dependent on multiple ligand interaction and difficult to distinguish any specific ligand-based function of NRP2. For that reason, in the current review, we did not separate the importance of NRP2 function based on the ligands.
1.1 Structure of NRP2
NRP1 and NRP2 share approximately 44% homology in amino acid sequence. Each has a large N-terminal extracellular domain (835 amino acid residues for NRP1 and 844 for NRP2), a short transmembrane domain (23 amino acids for NRP1 and 25 for NRP2), and a small cytoplasmic tail (44 amino acids for NRP1 and 42 for NRP2). The extracellular portion contains two CUB (complement binding homology) domains named a1 and a2 and two coagulation factor (V/VIII) homology domains called b1 and b2. In addition, a MAM (meprin, A-5 protein, and receptor protein-tyrosine phosphatase mu) domain, termed c, separates the b2 and transmembrane domains [5]. The cytoplasmic tail contains a PDZ-domain-binding motif. This motif is responsible for binding synectin (GIPC1), which bridges NRPs and a myosin 6-driven cell transport machinery for endocytic trafficking and activates small GTPase-activating proteins. Particularly, the last three amino acids of the c-terminal domain appear to be responsible for this interaction with small GTPase-activating proteins [7, 8]. The two NRPs differ in their specificity to ligands. Whereas NRP1 works as a receptor for semaphorin-3A, 3C, and 3F, NRP2 shows preferential binding to semaphorin-3B, 3C, 3D, and 3F [9,10,11,12]. In addition, NRP1 binds to vascular endothelial growth factor-A (VEGF-A), whereas NRP2 binds to VEGF-C and –D [13,14,15,16]. Due to the variety of structural domains present, NRPs can interact with several binding partners and initiate different signaling pathways. A schematic representation of the NRP2 structure is shown in Fig. 1. The protein has been depicted in crouch conformation based on our in silico observations after molecular modeling and simulation studies (our unpublished data).
Schematic representation of NRP2 structure and isoforms. The extracellular (A1-A2-B1-B2-C), transmembrane, and cytosolic domains are shown from top to bottom. From left to right: three (03) isoforms of NRP2, viz., NRP2A, NRP2B, and soluble NRP2 (s9NRP2), are presented with a depiction of their respective structural similarities and dissimilarities. The name of the domains and their canonical functions are displayed in the text box
1.2 NRP2 Isoforms
NRP2 has several alternative splice variants. Among them, NRP2A and NRP2B have been well studied. These two isoforms differ mainly in the linker region between the transmembrane and the cytoplasmic domains. There is 44% of sequence homology between NRP1 and NRP2A, whereas NRP2B resembles NRP1 by only 11% at the transmembrane and cytoplasmic domains. NRP2A is further characterized by an insertion of 17 or 22 amino acids following amino acid 809. They are known as NRP2A17 and NRP2A22, respectively. On the other hand, after amino acid 808, NRP2B contains 0 or 5 amino acid insertion, known as NRP2B0 and NRP2B5, respectively. In addition to NRP2A and NRP2B, a soluble isoform, known as s9NRP2, has also been identified [11, 17]. It is produced by the inclusion of an intron containing an in-frame stop codon before the transmembrane domain in the second coagulation factor domain (b2) and, thus, secreted. Therefore, s9NRP2 contains a1, a2, b1, and a portion of the b2 domain; however, it does not have MAM, transmembrane, and cytoplasmic domains. Thus, s9NRP2 can sequester NRP2 ligands such as VEGF-C and often exerts tumor inhibitory function [8, 18]. Increased secretion of soluble NRP2 was thus detected by alveolar macrophages upon lipopolysaccharide inhalation, which regulates the secretion of various pro-inflammatory cytokines [19]. A schematic representation of NRP2 isoforms is shown in Fig. 1.
1.3 NRP2 in normal physiology
NRP2 is primarily expressed on sympathetic neurons [20] and in venous and lymphatic endothelial cell (EC) [4, 21]. NRP2 regulates axon guidance cues in neurons by binding to the semaphorins (especially class III) through its a1 and a2 domains. Semaphorin 3F (Sema 3F) by interacting with the NRP2-expressing axons at the growing nerve tips has been shown to induce the repulsion of neuronal growth cones [2, 22,23,24]. NRP2 thus participates in the migration of neural crest cells [25] and is also required to properly develop several cranial and spinal nerves [22]. During postnatal development of cortical pyramidal and hippocampal neurons, Sema3F once again interacts with NRP2 and its co-receptor PlexA3 to regulate the apical dendritic spine morphogenesis and mediates excitatory synaptic transmission [24, 26]. NRP2 in endothelial cells actively participates in the process of angiogenesis, lymphangiogenesis, and vasculogenesis by interacting through its b1 and b2 domains with different VEGFs. NRP2 mRNA levels are significantly higher in endothelial tip cells than non-tip cells [27]. Multimeric transcription complex of GATA-binding protein 2 (GATA2) and LIM domain only 2 (Lmo2) positively regulates the NRP2 expression in endothelial cells by binding at GATA [28]. Paxillin also regulates the NRP2 expression and thus controls EC motility and invasion [29]. Moreover, under stress conditions, such as ischemia, NRP2 levels are upregulated in ECs [4, 30]. A reduction in the proper initiation of vascular sprouting upon NRP2 knockdown highlights its importance in VEGFA-mediated angiogenesis [31]. NRP2 thus promotes angiogenesis by increasing the VEGFA binding to VEGFRs [14, 28, 32]. On the other hand, transcription factor, Prox-1, while promoting the differentiation of venous EC into lymphatic EC, also increases the NRP2 levels [4, 33] in lymphatic ECs. NRP2 promotes lymphatic development, when stimulated by VEGF-C and VEGF-D and acts as a co-receptor for VEGFR-3 [6, 13, 16, 28]. The lack of small lymphatic vessels and capillaries is therefore observed in NRP2 null mice [21]. Other than neuronal cells and ECs, NRP2 is also expressed in the islet cells of the pancreas [4, 34], bladder urothelium [35, 36], and keratinocytes [37], the normal ovarian epithelium [6, 38]. In the gastro-intestinal tract, it is expressed in the colonic epithelium [39, 40], where it co-localizes with the enteroendocrine cells expressing chromogranin-A (CgA) [31, 39]. NRP2 expression is also observed in cells of the monocyte-macrophage family, including alveolar macrophages [41]. NRP2 expression has been further detected in both the osteoblasts and osteoclasts in bone and regulates the homeostasis of bone architecture [42]. NRP2 expression is regulated by several factors (Fig. 2a).
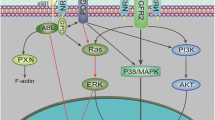
Schematic representation of NRP2 expression regulation and downstream signaling. a NRP2 expression regulation. The factors regulating the NRP2 expression in physiological and pathological conditions are depicted here. In physiological condition forskolin suppresses, while Prox1, GATA2, and Paxillin upregulates its levels. In cancer, TGFβ upregulates, while Wnt antagonist, Pax8, miRNAs, DLX2 decreases its expression. In ischemia, its levels are found to be elevated where HIF could play a role. b Proposed schematic diagram of NRP2 signaling pathway. NRP2 interacts with different ligand molecules and mediates the downstream signaling cascade. They are necessary for the semaphorin/plexin mediated signaling, while they act as co-receptors for VEGFR, c-Met, PDGFR, FGFR, Integrins, and TGFβ to enhance their signaling. I It interacts with semaphorin and Plexin to regulate axon guidance cues, synaptic transmission, and inhibit tumor progression. II It interacts with the VEGF and promotes angiogenesis, lymphangiogenesis, vasculogenesis and activates ERK/PI3K/p38/Src to promote tumor progression. III It interacts with c-Met/PDGFR/FGFR and promotes tumor progression via ERK/PI3K/p38/Src mechanism. IV It interacts with integrin and via FAK-Ras/MEK-Gli1 pathway to promote tumor progression. V It interacts with TGFβ to promote EMT via SPP1/OPN/Osteopontin and promotes tumor progression via activated Smad signaling. The orange boxes indicate their physiological function and gray boxes indicate their cancer-specific function
1.4 NRP2 in cancer
The elevated levels of NRP2 are often associated with cancer progression and poor prognosis in many cancers such as prostate cancer, osteocarcinoma, salivary adenoid cyctic carcinoma, breast cancer, neuroblastoma, and non-small cell lung cancer [43,44,45,46,47,48]. Several molecular mediators have been reported to enhance NRP2 expression in cancer (Fig. 2a). One of the key players in tumor-mediated NRP2 expression is transforming growth factor–β (TGFβ). In hepatocellular carcinoma, the increased TGF-β/Smad signaling leads to the NRP2 upregulation [49]. Mechanistically, TGF-β1 promotes histone modifications at the NRP2 promoter and thus induces expression [50]. A study in non-small cell lung cancer (NSCLC) cell lines further confirmed the role of TGF-β signaling in increasing the levels of NRP2. Interestingly, the study suggested that TGF-β induces the expression of specific NRP2b isoform, which is required for TGF-β mediated epithelial-mesenchymal transition in NSCLC [51].
Of late, studies have highlighted the role of microRNAs (miR) in regulating the levels of NRP2 under disease conditions. miR-331-3p is significantly downregulated in triple-negative breast cancer (TNBC) [52] and glioblastoma (GB) [53]. NRP2 gene presents a binding site of miR-331-3p in its 3′-untranslated region, and overexpression of miR-331-3p decreased its levels [52]. Thus, a decrease in miR-331-3p is essential for increasing NRP2 in TNBC and GB. Similarly, in the breast cancer cell line (MDA-MB-468), miR-146a-NRP2 axis promotes tumor aggressiveness [54]. miR-146a targets the 3′UTR of NRP2 at two binding sites and reduces NRP2 expression. Furthermore, miR-146a also inhibits the activity of NRP2 ligand, SEMA3C, by repressing the SEMA3C-induced invasion and proliferation [54]. miR-196a-3p also regulates NRP2 levels. In breast cancer cells, miR-196a-3p is significantly downregulated, which upregulates NRP2 expression leading to tumor progression [55]. In colorectal carcinoma (CRC), miRs play a crucial role in regulating the NRP2 expression. Overexpression of miR-486-5p in CRC cells reduced the expression of NRP2 and thus could inhibit tumor growth and lymphangiogenesis in nude mice, suggesting miR-486-5p suppresses CRC via NRP2 downregulation [56]. Since X-inactive specific transcript (XIST), which is upregulated in CRC, negatively controls the expression of miR-486-5p, it can be assumed that the XIST/miR-486-5p-mediates the NRP2 regulation, affecting the CRC progression [56, 57]. In gastric cancer, increased expression of NRP2 is regulated by miR-224-5p that correlates with disease progression. miR-224-5p expression is regulated by circular RNA low-density lipoprotein receptor class A domain containing 3 (circ-LDLRAD3). circ-LDLRAD3 levels are elevated in GC tissue samples, which suppresses miR-224-5p levels, thereby promoting NRP2 expression [58].
Several other factors are also involved in the NRP2 regulation, such as distal-less homeobox 2 (DLX2), Wnt signaling, and Pax8. DLX2 plays a critical role in cellular differentiation, proliferation, migration, pattern formation, and neurogenesis [59,60,61,62], and it gets downregulated in solid and hematologic malignancies and lymphoblastic leukemias [63,64,65]. Repression of DLX2 by p53-R273H causes increased NRP2 expression in the NSCLC, human hepatocellular carcinoma, and human TNBC cell lines [66]. In osteosarcoma cell lines, Wnt antagonists negatively regulate NRP2 expression. It is observed that secreted Wnt antagonists, such as Wnt-inhibitory factor-1 (WIF-1), secreted Frizzled-related proteins (SFPs), and soluble low-density lipoprotein receptor-related protein 5 (sLRP5), significantly decrease the NRP2 mRNA and protein levels [67]. In thyroid carcinoma tissues and cell lines, an inverse correlation exists between the expression of PAX8 and NRP2. When Pax8 was ectopically overexpressed, NRP2 was downregulated, resulting in reduced cell proliferation, migration, and invasion [68].
1.5 The tumor cell-specific function of NRP2
Several reports have indicated towards the direct association between high NRP2 expression and the tumor progression [43, 69,70,71]. NRP2 often modulates cellular communication and migration of cancer cells by acting as co-receptor not only for the Plexins (receptors for Sema) and VEGF receptor families but also for transforming growth factor (TGF) receptors, hepatocyte growth factor (HGF) receptor, and platelet-derived growth factor (PDGF) receptor [72,73,74]. Interestingly, for many cancer types, VEGF and Sema family ligands have opposite effects on the NRP2 axis in the development and progression of tumors. Whereas VEGFs have a tumor-promoting function, Semas confer inhibitory signals on tumorigenesis [75,76,77,78]. Sema, especially Sema-3B and Sema-3F, interacts preferentially with NRP2 and confer inhibitory signals to cancer cell migration and metastasis [75,76,77,78]. Although multiple signaling pathways are involved in Sema-mediated inhibition of NRP2-axis, the reduction of Akt phosphorylation is commonly observed [79, 80]. Activation of NRP2 axis by HGF and fibroblast growth factor (FGF) potentiates the induction of cancer cell proliferation, survival, and invasion [81] — a function once again inhibited by Sema. Mechanistically, it has been shown that NRP2 can increase the phosphorylation and, thus, activate the c-Met receptor (canonical receptor for HGF) either by binding to HGF or by interacting directly with the receptor [82]. Through c-Met, NRP2 activates different signaling pathways, including ERK, PI3K, p38-MAPK, and Src, and contributes to tumorigenesis [81,82,83]. Similarly, NRP2 phosphorylates the receptors of PDGF, both PDGFRα and PDGFRβ, to increase PDGF receptor-mediated cell proliferation and migration [84]. NRP2 by associating with TGF-receptor I and II (TGFRI and TGFRII) can phosphorylate and thus activate Smad 2/3, which aids in tumor progression [85]. There is an alternative way NRP2 can activate TGFRs. It does by helping to release the TGFβ1 (active form) from its inhibitory complex with the latency-associated peptide (LAP). NRP2’s b2 domain contains a motif of basic peptides (RKFK). Closely related 94RKPK peptide of TGFβ1 binds to LAP to form the latent LAP-TGFβ1 pro-protein complex. Classically, this pro-protein latent complex needs to be cleaved within the trans-golgi to yield mature TGFβ1. The basic RKFK peptide motif of NRP2 b2 domain competes with the TGFβ1 94RKPK peptide for binding to LAP. Thus, NRP2 helps to increase the mature functional TGFβ1 [86]. NRP2/TGFβ1 axis has been shown to play an active role in the epithelial to mesenchymal transition (EMT) of cancer cells [87]. EMT can lead to cancer cell invasion, migration, and metastasis [88]. Several epithelial markers (such as keratin 20 and E-cadherin) are decreased along with an increment in mesenchymal markers (for example, vimentin) in cancer cells in the presence of elevated NRP2 [87]. Moreover, NRP2 was shown to regulate β-catenin signal to drive EMT and has been linked to enhancing the expression of metastatic and anti-apoptotic genes as well as the development of therapy resistance to chemotherapeutic agents [89]. A detail discussion on tumor cell–specific role of NRP2 has been discussed in the following section, where we have discussed the function of NRP2 axis in individual cancer type.
1.6 The EC-specific function of NRP2
VEGF/NRP2 axis in vascular and lymphatic ECs leads to lymphangiogenesis/angiogenesis through interactions with VEGFRs, especially VEGFR2 and VEGFR3 [14]. NRP2 increases the ligand-induced phosphorylation of VEGFRs. This subsequently leads to the activation of ERK/PI3K pathways and contributes to angiogenesis and/or lymphangiogenesis, leading to metastasis of tumors [90, 91]. Furthermore, the survival of human microvascular ECs is also increased upon stimulation by VEGF-C or VEGF-A when NRP2 is overexpressed. Therefore, elevated NRP2 levels in the EC are responsible for increased responsiveness to VEGF and a more aggressive cancer phenotype [92]. NRP2 also helps VEGFRs to interact with integrins and promotes the migration of ECs over the extracellular matrix (ECM) [14, 93]. Mechanistically, NRP2 regulates the recycling of α5 integrin (ITGA5, a fibronectin-binding integrin) in Rab11 dependent manner and promotes the ITGA5-mediated adhesion and migration of ECs over fibronectin — a mechanism quite distinct from NRP1 that depends on β3 integrin (ITGB3). Interestingly, this mechanism of EC migration is independent of NRP2’s function as a co-receptor of VEGFRs [93].
1.7 Immune cell-specific function of NRP2
NRP2 in the tumor resident immune cells play a role in active tumor progression. We observed that NRP2 is highly expressed in tumor-associated macrophages (TAMs) during macrophage differentiation [71]. NRP2 depletion was found to affect phagosome maturation during phagocytosis. NRP2-expressing macrophages in the tumor microenvironment thus regulate the phagocytosis of tumor cells, a process known as efferocytosis. Enhanced efferocytosis in the tumor microenvironment promotes immune suppression and thus favors tumor growth. Depletion of NRP2 from the TAMs thus inhibits efferocytosis and increases secondary necrosis within the tumors by hindering the process of apoptotic tumor cell clearance. These results to an enhanced infiltration of natural killer and CD8 + T cells and thus can facilitate anti-tumor immune response [71]. Such the role of NRP2 in immune regulatory processes opens a new horizon of a novel immunomodulatory pathway stimulated by the tumor cells to evade immune detection within the host and underscores the importance of targeting NRP2 axis in macrophages for enhancing anti-tumor immune response. A detail discussion on NRP2’s role in immune cells was discussed elsewhere [94].
All these results therefore suggested an active NRP2 axis both in tumor cells and in the tumor microenvironment, which can promote tumor growth and metastatic progression. The functional activity of NRP2 is depicted in Fig. 2b.
1.8 NRP2 in different cancers
NRP2 expression is found to be upregulated in several cancer types and is associated with cancer progression, metastasis, therapy resistance, and poor prognosis [43, 69, 95]. In the following section, we have discussed the NRP2 expression status and its cancer promoting roles in each cancer type:
1.9 Genitourinary cancers
1.9.1 Prostate cancer (PCa)
In a study conducted on tissue samples from primary PCa patients, 68% were found to be positive for NRP2 staining. Thirty-three percent of NRP2 positive were of high-risk PCa, 34% with a GS ≥ 8, and 31% exhibited locoregional lymph node metastasis. Moreover, the overall survival rate of patients positive for NRP2 is lower as compared to patients negative for NRP2 [43]. In a separate study, NRP2 expression was evaluated in human primary PCa and bone metastatic PCa tissues. While a heterogeneous expression of NRP2 was detected in primary PCa, a strong homogenous expression of NRP2 was detected in 85% of bone metastatic tissues [96, 97]. Analysis of RNA-seq data of the stand-up-to-cancer cohort also suggested a significantly higher level of NRP2 in metastatic PCa, especially in bone [98]. Another study indicated increased expression of NRP2 in high-grade PCa (Gleason grade 5) and metastases when compared with low-grade PCa (Gleason grade 3). All these studies thus suggested functional involvement of NRP2 during the progression of PCa, especially during metastasis [95]. Direct evidence of NRP2’s role in PCa came from animal models of PCa. NRP2 inhibition in the xenograft model of PC3 cell lines showed significant tumor growth inhibition [95]. In an intratibial model of PCa, it was observed that NRP2 depletion significantly enhanced the sensitivity of LNCaP C4-2B cells to taxane-based chemotherapy [96]. Several in vitro studies have also suggested the role of NRP2 in PCa cell proliferation, EMT, and their survival during therapy [70, 99].
Mechanistic studies have been performed to explain the tumor-promoting roles of NRP2 in PCa. During oxidative stress, NRP2/VEGF-C axis was found to be necessary to maintain mTOR complex 2 (mTORC2) in a functional state. AKT-1, the downstream mTORC2, thus can retain its activity and promote survival of NRP2-expressing PCa cells [69]. VEGF-C/NRP-2 axis also facilitates autophagy during nutrient deprivation or during therapeutic stress and thus helps PCa cells to evade chemotherapeutic stress [99]. Additionally, endocytic transport of cell surface epidermal growth factor receptor (EGFR) was affected by NRP2 depletion, resulting in cell death [70]. As an underlying mechanism, accumulation of early endosomes marked by EEA1/Rab5-positive expression was observed upon NRP2 depletion leading to reduction of Rab7-positive late endosomes, thereby affecting early-to-late endosome maturation. This defect in early to late endosome maturation upon NRP2 inhibition could thereby influence aberrant recycling of EGFR through the endocytic pathway and hinder the maturation of autophagosome to autolysosome [70]. It is important to note that autophagosomes often share the cellular machinery with late endosomes to fuse with the lysosome. A decrease in late endosomes due to the absence of NRP2 can thus affect the autophagy process. It was further observed that a protein called WD-repeat and FYVE domain-containing protein 1 (WDFY1) acts downstream of the VEGF-C/NRP-2 axis to regulate the endocytic pathway in PCa cells. VEGF-C/NRP2 negatively regulates the expression of WDFY1 by preventing the nuclear translocation of the transcription inhibitor, Fetal ALZ50-reactive clone 1 (FAC1) in PCa cells [99, 100]. An increased level of WDFY1 in the absence of NRP2 thus affects the transition of early to late endosomes. Further studies have been performed to understand how NRP2 regulates the expression of some specific genes. It was observed that NRP2 can translocate to the nuclear membrane upon ligand activation through a retrograde mechanism and can form complex with nuclear pore proteins (NUPs). The complex of NRP2 and NUPs can then recruit specific transcription factors to the promoter region of genes, which are important for tumor progression [101]. Androgen receptor is one of the important transcription factors that can be recruited to the promoter regions of many cancer-promoting genes through this NRP2-Nup complex.
Apart from the association of NRP2 expression with prostate cancer grade and clinical outcome, its high expression also correlates with PTEN loss. PTEN-null prostate cancer induces NRP2 upregulation, which in turn is responsible for Bmi-1- (a polycomb group transcriptional repressor) mediated repression of the insulin-like growth factor 1 receptor (IGF-1R) [95]. NRP2 expression is also associated with the therapy resistance against VEGF axis inhibitors. VEGF signaling in tumor cells is an essential mediator of tumor initiation and progression, and it is targeted by drugs such as bevacizumab and sunitinib for cancer therapy. However, NRP2 upregulation confers resistance to PCa cells against these drugs by upregulating the P-Rex1, which leads to the activation of Rac1/ERK signaling cascade [102].
Recently, NRP2 has been detected in therapy resistant prostate cancer cells with neuroendocrine like characteristics (t-NEPC) [103]. Interestingly, the secretion of several cytokines and growth factors such as IL8, VEGF, and angiopoietin in tNEPC cells is under the control of NRP2 axis. Many of these cytokines/growth factors act in both autocrine and paracrine manner to promote resistance to therapy not only to the neuroendocrine-like prostate cancer cells but also to the adjacent adenocarcinoma cells present in the same cancer tissue.
1.9.2 Bladder cancer
NRP2 upregulation in bladder cancers (BLCa) is also reported. High NRP2 expression is observed in BLCa and is associated with a poor prognosis. It is closely associated with the tumor size, stage, and grade. High NRP2 expression correlates with the overall survival (OS) of cancer patients. Mean OS for high NRP2 expression was found to be 47 months as compared to 94 months for low NRP2 expression. The study thus presents NRP2 expression as a predictive marker for patient outcome, where it is responsible for a 3.85-fold increase in cancer-specific deaths [104]. NRP2 expression can thus be a valuable prognostic factor, especially for muscle-invasive, and high-risk BLCa patients, and therefore, it could be targeted along with radiochemotherapy to manage these patients. In another study, the expression of NRP2 and its transcript variants correlate with clinical outcome in BLCa, where NRP2 expression combined with a high NRP1, PDGFC, or PDGFD expression is associated with reduced OS. The study also proposed that NRP2A, a specific splice variant, is an independent prognostic marker for a shorter cancer-specific survival (CSS) in BLCa patients [105]. A separate study further indicated that NRP2’s expression in BLCa negatively correlates with that of immune checkpoint gene, Sialic acid-binding Ig-like lectin 15 (SIGLEC15), and is responsible for the depletion of CD4 + T cell central memory [106]. It has been reported that the expression of NRP2 in BLCa could be regulated by TGFβ1. Cell line data further suggested that NRP2 can promote EMT in BLCa cells by enhancing the levels of secreted phosphoprotein 1 (SPP1/OPN/Osteopontin) [107].
1.9.3 Renal cancer
NRP2 is considered a risk factor in kidney renal papillary cell carcinoma (KIRP) and kidney renal clear cell carcinoma (KIRC). High NRP2 expression in KIRP is associated with poor prognosis [106, 108]. NRP2 expression is negatively correlated with the tumor mutational burden (TMB) and is responsible for the depletion of T cell CD4 + central memory. NRP2 expression is also positively associated with microsatellite instability (MSI) in KIRC [106]. In a retrospective study with advanced-stage renal cell carcinoma (RCC), 49% showed high expression of NRP2 by tissue immunostaining. Progression-free survival (PFS) for RCC patients with high NRP2 expressing cancer was 4 months as compared to 11 months for patients with low NRP2 expressing tumors. Similarly, median OS was longer for low NRP2 expression (26 months) as compared to high NRP2 expression (13 months) [109]. NRP2 expression was elevated in metastatic carcinoma as compared to matched primary tumors in RCC, suggesting its potential involvement in the metastatic progression. In the mouse model of RCC, NRP2 is found to promote lung metastasis. An in vitro study with RCC cell (786-O) and endothelial cell (HUVEC) proved that NRP2 expression in tumor cells enhanced their adhesion to ECs by interacting with α5 integrin present on ECs and facilitating extravasation [108].
1.10 Gastrointestinal (GI) cancers
1.10.1 Gastric cancer
Immunohistochemical staining of tissues of human gastric cancer patients confirmed the NRP2 expression in gastric cancer epithelium, while normal mucosal epithelium does not have NRP2 expression [89, 92]. Knocking down NRP2 in GI cancer cells reduced β-catenin activity, leading to a decreased metastatic gene expression and reduced migration and invasion of the cells. Furthermore, NRP2 knockdown made the cells vulnerable to chemotherapy [89]. High NRP2 levels in tumor cells also upregulate NRP2 expression in surrounding ECs, which thus promotes enhanced angiogenic phenotype of the gastric tumor by promoting VEGF-induced proliferation and migration of ECs [92].
Studies on the small intestinal neuroendocrine tumors (SI-NETs) patients revealed a NRP2 upregulation in tissue and plasma samples. In tissue, the presence of membranous NRP2 expression correlates with invasion, metastatic abilities, and neovascularization. Additionally, elevated serum levels of NRP2 soluble isoform are observed in metastatic patients. In-depth analysis in preclinical mouse models of SI-NET suggested that knockdown of NRP2 imparted antitumor effects by VEGFR2 downregulation. NRP-2 inhibition led to decreased tumor cell viability and higher susceptibility towards therapeutic agents [110].
1.10.2 Liver cancer
NRP2 has also been observed to be upregulated in liver malignancies. In a study conducted on hepatocellular carcinomas (HCC) patients, NRP2 expression was observed to be increased in higher grades and correlates with poor prognosis [49]. Moreover, NRP2 expression was high in HCC patients with cirrhosis (61.1%) as compared to those without (38.9%) [111]. Interestingly, NRP2 expression coincides with that of TGF-β1 in patient samples and HCC cell lines. The study further showed that NRP2 expression is upregulated by TGF-β/Smad signaling, and inhibition of TGF-β signaling or NRP2 knockdown reduced the cell migration [49].
NRP2 expression is also observed in hepatoblastoma (HB) patients and HB cell lines. NRP2 silencing in HB cell lines decreased their viability and their migration, indicating its indispensable role in HB progression [112].
1.10.3 Pancreatic cancer
NRP2 expression was upregulated in human pancreatic ductal adenocarcinoma (PDAC) tissue specimens, which is associated with poor prognosis [91, 113, 114]. It is also highly expressed in the pancreatic cancer cell lines, where NRP2 knockdown decreased their migration and invasion [91, 113]. Moreover, in the murine subcutaneous xenograft model, knockdown for NRP2 had a lower proliferation rate, produced tumors with low volume, and altered the vasculature [91]. Further studies established that decreased expression of NRP2 in PDAC caused abnormal activation of ERK and caused premature cell death. This process is usually employed by pancreatic cancer cells to evade drug action [70]. Wang et al. developed a mouse monoclonal antibody against NRP2 (N2E4) that could efficiently bind to PDAC cells. The study confirmed that N2E4 inhibited the PDAC proliferation, migration, invasion, and metastasis, via blocking NRP2-integrinβ1 interaction [114]. The study, therefore, presented a background where NRP2 targeted therapy suppresses PDAC progression. Another study explored the role of IL-8 in tumor progression and proliferation in PDAC. IL-8 was found to be upregulated in the pancreatic cancer cells that caused over-expression of VEGF-C and NRP2 in cancer cells [115]. In a separate study, it was reported that mucin 16 can promote liver metastasis of pancreatic ductal adenocarcinoma by upregulating NRP2 [116]. The presence of NRP2 is also observed in endocrine pancreatic tumors [34]. The role of NRP2 in pancreatic cancer is also extended in the tumor-associated macrophages (TAM). Tumor cells induce NRP2 expression in macrophages while they differentiate, which regulates their phagocytic function. This NRP2 expression in TAM promoted tumor growth by promoting efferocytosis, and NRP2 knockdown increased the secondary necrosis within tumors. Overall, the results suggest that targeting NRP2 in TAM initiates antitumor immune responses by increased infiltration of CD8 + T and natural killer cells [71].
1.10.4 Colorectal cancer
Evidenced by multiple studies, the NRP2 expression shows upregulation in both human primary and metastatic colorectal cancer. In a study, its expression was found in 83% of adenocarcinomas tissue samples and in 71% of liver metastases [90]. Another study also observed similar NRP2 protein expression in patient tissue samples, 38% in primary and 91% in metastatic cancer [117]. It is also found to be expressed in the colorectal cancer cell lines [90]. Cell line models have shown that NRP2 promotes a better survival advantage for cancer cells under both anchorage-independent and hypoxic conditions. The role of VEGFR1 in mediating cellular migration and invasion was more pronounced in effect in the presence of NRP2 [90]. Substantial studies have reported the critical involvement of NRP2 in promoting invasive phenotype in colorectal cancers. NRP2 involvement has been shown to be associated with developing mesenchymal properties concurrent with vimentin expression. The cross-talk between NRP2 and TGF beta1 induces constitutive activation of Smad complexes, at the same time reducing inhibitory Smad 7 signaling in colon cancers. This molecular network shows a coordinated role of NRP2 co-receptor through TGF beta-Smad signaling to initiate the EMT transition, thereby implying a potential function in tumor cell migration [87]. Thus, NRP2 plays concurrent roles in promoting survival, invasion, and migration in colorectal cancer progression.
1.11 Cancers of the central nervous system
1.11.1 Glioma
In the tissue samples from glioblastoma (GB) patients, NRP2 expression is observable in both the cytoplasm and membrane. NRP2, along with VEGF-C, is highly upregulated in these patients and is associated with poor prognosis, making NRP2 an important prognostic predictor in glioblastoma. Interestingly, patients with a low level of NRP2 responded to therapy much better than those with a higher level. It, therefore, suggests that the VEGF-C/NRP2 axis can be a potential drug target in glioblastoma [118]. NRP2 expression is also high in GB cell lines [118], and NRP2 levels regulate the proliferation, clonogenic growth, and migration of tumor cells [53]. Abnormal expression of miR-331-3p has been found to play an inhibitory role in the initiation and advancement of high-grade glioma. Interestingly, it exerts its effect by downregulating NRP2 corroborating the oncogenic role of NRP2 in high-grade gliomas [53].
In vivo studies further demonstrated that the downregulation of NRP2 by miR-15b and miR-152 significantly reduced the invasion potential of glioma tumors via deactivation of the MEK/ERK pathway [119]. In a study conducted by Nassare et al., it was observed that the chemorepulsive effect of Sema3A can be modulated by NRP2, which can convert a chemorepulsive effect into a chemoattractive response [120], thus providing a mechanism of how NRP2 promotes migration and invasion of glioma cells through a chemotactic gradient.
1.11.2 Neuroblastoma
NRP2 was detected in tumor biopsies of neuroblastoma (NB) patients and correlated with the tumor stages. However, the immunohistological analysis revealed that ECs and not the neuroblastoma cells were expressing high NRP2 levels. In the early stages, its expression was limited to the capillaries and in postcapillary venules. In contrast, in advanced stages, it is found to be expressed in mid-sized and larger vessels [48]. However, detailed studies are needed to fully understand the role of NRP2 in NB progression.
1.12 Breast cancer
NRP2 expression is elevated in human invasive breast cancer (BrCa) [121], including triple-negative breast cancer (TNBC) [122]. VEGF/NRP2 signaling pathway is essential for the sustained proliferation and survival of the breast cancer stem cells (CSCs), promoting tumor progression [123]. In BrCa tissues, NRP2 expression significantly correlates with the expression of cytoplasmic CXC chemokine receptor-4 (CXCR4), VEGF-C, and lymph node metastasis. Analysis of human BrCa patients revealed that the higher NRP2 expression was associated with poor overall survival. It was identified as a significant independent predictor for the long-term survival of BrCa patients. Moreover, in vitro experiments using human BrCa cell lines demonstrated that cytoplasmic CXCR4 expression and CXCR4-mediated migration of human BrCa cells was inhibited following NRP2 silencing with neutralizing antibody [47]. NRP2 is highly expressed in several BrCa cell lines, and blocking NRP2 affected their proliferation and migration [121]. Increased NRP2 is required for adherence of the BrCa cells to laminin matrices. NRP2 helps in the process by forming more focal adhesions on laminin by regulating α6β1 integrin and promoting α6β1 integrin-driven activation of the FAK/Src signaling pathway. NRP2 is also necessary for the association of α6β1 integrin with the cytoskeleton and for cancer cell migration and metastasis [124]. Moreover, α6β1 integrin and FAK-driven propagation of Ras/MEK signaling cascade consequently upregulates the expression of Hedgehog signaling effector, Gli1, and activates an important stem cell factor, BMI-1. This again enhances the NRP2 production and its interaction with α6β1 integrin, establishing an autocrine loop. In addition, NRP2 depletion delays the initiation of TNBC, as demonstrated in in vivo model [122]. In a separate study, it was shown that VEGF/NRP2 axis protects the TNBC cells from DNA-damaging chemotherapeutic agents (platinum-based chemotherapies) by promoting homologous recombination (HR) to achieve an efficient DNA double-strand break repair. NRP2 does so by upregulating the expression and function of the central enzyme in the HR pathway, Rad51, in a YAP/TAZ-dependent manner [125].
1.13 Melanoma
NRP2 is expressed in melanoma. Immunohistology data from melanoma patients revealed 86% samples of primary tumor and 90% samples of metastatic tumor were positive for NRP2 expression (> 20% staining) [126]. Moreover, NRP2 transcript levels were also observed to be high for melanoma patients, where the expression was increased from primary to metastatic melanoma [127]. NRP2 expression was also detectable in the Spitzoid malignant melanoma (SMM), but the small sample size of the study calls for further studies to establish the NRP2 association with SMM [128]. Rapid EMT occurs during the early stages of metastasis of melanomas that is attributed to the NRP2, which facilitates efficient cross-talk between melanoma and ECs in the tumor microenvironment [129]. NRP2 silencing in metastatic melanomas suppressed the tumor cell growth and, in the mouse model, inhibited the tumor formation and its metastasis, while its upregulation promoted tumor formation [18, 127, 129, 130].
1.14 Other cancers
1.14.1 Hematological cancers
NRP2 is found to be highly expressed in different types of myeloid leukemias, including acute myeloid leukemia (AML), chronic myeloid leukemia (CML), chronic eosinophilic leukemia (CEL), chronic myelomonocytic leukemia (CMML), or mast cell leukemia (MCL) [131]. T-cell acute lymphoblastic leukemia cells also express NRP2, which modulates the migration of malignant cells [132].
1.14.2 Thyroid cancer
Thyroid papillary carcinoma tissue samples express high levels of NRP2 [133, 134]. Transcription factor PAX8 activity has been reported to be important for high NRP2 expression in thyroid cancer [68, 135]. A study on cancer tissue samples found high NRP2 expression in 54.2% of the patient samples that correlated with high lymph node metastasis [133]. NRP2 knockdown suppresses the migration, invasion, and EMT in this cancer type [68, 133, 136].
1.14.3 Lung cancer
NRP2 is expressed in the lung cancer tissue samples including, non-small cell lung carcinoma (NSCLC) [137]. In a study on NSCLC specimens, 64.7% were positive for NRP2 that correlated with poor prognosis [45]. Lung cancer cell lines also express high NRP2 levels [138, 139]. NRP2 expression is driven by the TGFβ1-mediated EMT, and suppressing NRP2 inhibits EMT, migration/invasion, ERK activation, and growth [139].
1.14.4 Bone cancer
NRP2 levels are high in osteosarcoma, a type of malignant bone tumor. NRP2 expression is regulated by Wnt signaling in this cancer [67]. High NRP2 expression enhances bone vascularity and is associated with a poorer prognosis [44]. Knockdown of NRP2 expression by short-hairpin (Sh) RNA resulted in reduced tumor growth, metastasis, and blood vessel formation of osteosarcoma. NRP2 suppression reduces the growth and proliferation of cancer cells.
1.14.5 Ovarian cancer
NRP2 expression is observed in the ovarian cancer tissue samples where its levels are significantly high in carcinomas compared to benign tumors [46]. Elevated levels of NRP2 are associated with tumor progression and poor prognosis [140].
2 Summary and future directions
NRP2 was initially discovered as a mediator in vascular and central nervous system development. However, it has been established to have important roles in tumor progression, metastasis, and therapy resistance over the years. As we have discussed throughout this review that high NRP2 expression is frequently observed in metastatic tumor tissues for several cancers and correlates with poor cancer-specific and overall survival of cancer patients. One common theme that is observed in several cancers is the ability of NRP2 axis to protect cancer cells from therapies. It protects the cancer cells by regulating the vesicular trafficking process in the cell and also by regulating the functions of several cell surface receptors such as VEGFRs, TGFRs, c-Met, integrins, and cytokine receptors and their downstream signaling axes such as Rac1/ERK, Gli1/BMI-1, and Fac. NRP2 axis also controls homologous DNA repair mechanisms and thus promotes therapy resistance. Studies have further indicated that NRP2 axis can promote metastasis by enhancing the ability of cancer cells to migrate or invade. Activation of β-catenin, CXCR4, and TGF-β signaling or upregulation of EMT program or facilitating integrin-mediated attachment of cells to extracellular matrix is some of the known mechanisms by which NRP2 axis regulates this migratory or invading potential of cancer cells. We have also highlighted in this review that NRP2 axis is active in several stromal cells and thus promotes tumor metastasis. Several studies have indicated that NRP2 axis in myeloid cells and lymphocytes promote immune suppression in tumor microenvironment. It is also well understood that NRP2 axis in endothelial cells is required for angiogenesis and helps the cancer cells to extravasate. These studies therefore highlighted the importance of NRP2 axis in tumor and stromal cells, which result in metastatic and therapy-resistant cancers. However, further studies need to be performed to understand whether the intracellular signaling regulation by NRP2 can influence the ensembles in the TME, and if so, what the detailed molecular mechanism is. As previously discussed, the NRP2 helps the advanced cancer cells to attain secretory phenotype by regulating vesicular transport and exocytosis. Thus, the cancer cells communicate with each other by secreting pro-tumorigenic growth factors and cytokines. Such paracrine communications create an environment in the tumor milieu to resist therapeutic pressure. To have a preliminary understanding, we have performed a network analysis of NRP2 using ingenuity pathway analysis (IPA) tool [141]. Interestingly, along with the canonical signaling network described above in this review, we observed NRP2’s association with players responsible for cellular adhesion, communication, vesicular secretion, and synaptic signaling regulation (Fig. 3). For example, on the cell surface, NRP2 interacts with neuronal cell adhesion molecule L1CAM that has important functions in neural development, synaptic plasticity, and signal transduction [142]. L1CAM is also involved in cancer progression, metastasis, and poor prognosis [143]. It can bind with integrin, neuropilins, FGFR, and other binding partners both in cis and in trans to establish a concerted intra- and inter-cellular communication and signal transduction to drive cancer cell motility, proliferation, and angiogenesis [143]. Similarly, NRP2 was found to interact with another neuronal cell adhesion molecule NRCAM, having canonical role in nervous system development, axon guidance, and synapse formation [144]. NRCAM is implicated in growth, proliferation, and progression of different cancers including neuroblastoma, thyroid, and prostate cancer [145,146,147]. On the other hand, NRP2 is associated with synuclein alpha (SNCA), extended synaptotagmin 1 (ESYT1), and focal adhesion kinase in the cellular cytoplasmic compartment (Fig. 3). These molecules control vesicular trafficking, secretion of cargo, and signal transmission in the extracellular space [148,149,150]. This observation from IPA-based interacting partner analysis warrants the investigation of NRP2’s comparatively unexplored role in regulating vesicular secretion, exocytosis, and signaling molecule release into the extracellular milieu. By doing above, it is possible that NRP2 helps to establish autocrine and paracrine communication among the cells present in the microenvironment, requiring further studies. In the context of cancer, direct cellular contact via the adhesion molecules as well as paracrine communication among the cells within the TME can lead to tumor proliferation, metastasis, and therapy resistance [151]. Therefore, it will be interesting to study whether and how NRP2 regulates such cellular communication and signal transduction to facilitate cancer progression and development of therapy resistance phenotype.
Proposed network of NRP2 with its interacting partners. Ingenuity pathway analysis (IPA) showing the network of interacting partners of NRP2. Partners are shown at the cellular compartments where the individual molecules are present: top, extracellular space; second from top, plasma membrane; second from bottom, cytoplasm; bottom, nucleus. NRP2 interacting proteins involved in cellular adhesion, communication, vesicular secretion, and paracrine signal transduction are highlighted in red
Besides, although there are numerous reports substantiating NRP2’s role in poor cancer prognosis, there is no effective inhibitor in the market to block NRP2 axis. A few efforts have been made to discover NRP2-targeted small molecule inhibitors [152, 153]. Among the compounds, benzamidine-based inhibitors that bind to the VEGF-C binding domain to competitively disrupt NRP2/VEGF-C interaction showed some promise in the plate-based binding assay [153]. However, further investigations need to be performed to validate their efficacy in cell line and animal models. In addition, aTyr pharma has developed peptide-based NRP2 inhibitor, ATYR1923, which is currently in phase 1b/2a clinical trial in pulmonary sarcoidosis and phase 2 trial in patients with COVID-19 related pneumonia (https://atyrpharma.com/programs/atyr1923). Nevertheless, it is necessary to continue the endeavors of drug development to achieve a safe and effective therapy against NRP2 axis.
References
York, J. R., Yuan, T., Lakiza, O., McCauley, D. W. (2018). An ancestral role for semaphorin3F-neuropilin signaling in patterning neural crest within the new vertebrate head. Development 145. (14). https://doi.org/10.1242/dev.164780.
Takahashi, T., Nakamura, F., Jin, Z., Kalb, R. G., & Strittmatter, S. M. (1998). Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nature Neuroscience, 1(6), 487–493. https://doi.org/10.1038/2203
Bae, D., Lu, S., Taglienti, C. A., & Mercurio, A. M. (2008). Metabolic stress induces the lysosomal degradation of neuropilin-1 but not neuropilin-2. Journal of Biological Chemistry, 283(42), 28074–28080. https://doi.org/10.1074/jbc.M804203200
Bielenberg, D. R., Pettaway, C. A., Takashima, S., & Klagsbrun, M. (2006). Neuropilins in neoplasms: Expression, regulation, and function. Experimental Cell Research, 312(5), 584–593. https://doi.org/10.1016/j.yexcr.2005.11.024
Pellet-Many, C., Frankel, P., Jia, H., & Zachary, I. (2008). Neuropilins: Structure, function and role in disease. The Biochemical Journal, 411(2), 211–226.
Wild, J. R., Staton, C. A., Chapple, K., & Corfe, B. M. (2012). Neuropilins: Expression and roles in the epithelium. International Journal of Experimental Pathology, 93(2), 81–103. https://doi.org/10.1111/j.1365-2613.2012.00810.x
Cai, H., & Reed, R. R. (1999). Cloning and characterization of neuropilin-1-interacting protein: A PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. Journal of Neuroscience, 19(15), 6519–6527.
Parker, M. W., Linkugel, A. D., Goel, H. L., Wu, T., Mercurio, A. M., & Vander Kooi, C. W. (2015). Structural basis for VEGF-C binding to neuropilin-2 and sequestration by a soluble splice form. Structure., 23(4), 677–687. https://doi.org/10.1016/j.str.2015.01.018
Kolodkin, A. L., Levengood, D. V., Rowe, E. G., Tai, Y. T., Giger, R. J., & Ginty, D. D. (1997). Neuropilin is a semaphorin III receptor. Cell, 90(4), 753–762. https://doi.org/10.1016/s0092-8674(00)80535-8
He, Z., & Tessier-Lavigne, M. (1997). Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell, 90(4), 739–751. https://doi.org/10.1016/s0092-8674(00)80534-6
Chen, H., Chedotal, A., He, Z., Goodman, C. S., & Tessier-Lavigne, M. (1997). Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron, 19(3), 547–559. https://doi.org/10.1016/s0896-6273(00)80371-2
Giger, R. J., Urquhart, E. R., Gillespie, S. K., Levengood, D. V., Ginty, D. D., & Kolodkin, A. L. (1998). Neuropilin-2 is a receptor for semaphorin IV: Insight into the structural basis of receptor function and specificity. Neuron, 21(5), 1079–1092. https://doi.org/10.1016/s0896-6273(00)80625-x
Karpanen, T., Heckman, C. A., Keskitalo, S., Jeltsch, M., Ollila, H., Neufeld, G., Tamagnone, L., & Alitalo, K. (2006). Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. The FASEB Journal, 20(9), 1462–1472. https://doi.org/10.1096/fj.05-5646com
Favier, B., Alam, A., Barron, P., Bonnin, J., Laboudie, P., Fons, P., Mandron, M., Herault, J. P., Neufeld, G., Savi, P., Herbert, J. M., & Bono, F. (2006). Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood, 108(4), 1243–1250. https://doi.org/10.1182/blood-2005-11-4447
Caunt, M., Mak, J., Liang, W. C., Stawicki, S., Pan, Q., Tong, R. K., Kowalski, J., Ho, C., Reslan, H. B., Ross, J., Berry, L., Kasman, I., Zlot, C., Cheng, Z., Le Couter, J., Filvaroff, E. H., Plowman, G., Peale, F., French, D., … Bagri, A. (2008). Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell, 13(4), 331–342. https://doi.org/10.1016/j.ccr.2008.01.029
Xu, Y., Yuan, L., Mak, J., Pardanaud, L., Caunt, M., Kasman, I., Larrivee, B., Del Toro, R., Suchting, S., Medvinsky, A., Silva, J., Yang, J., Thomas, J. L., Koch, A. W., Alitalo, K., Eichmann, A., & Bagri, A. (2010). Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. Journal of Cell Biology, 188(1), 115–130. https://doi.org/10.1083/jcb.200903137
Rossignol, M., Gagnon, M. L., & Klagsbrun, M. (2000). Genomic organization of human neuropilin-1 and neuropilin-2 genes: Identification and distribution of splice variants and soluble isoforms. Genomics, 70(2), 211–222. https://doi.org/10.1006/geno.2000.6381
Geretti, E., van Meeteren, L. A., Shimizu, A., Dudley, A. C., Claesson-Welsh, L., & Klagsbrun, M. (2010). A mutated soluble neuropilin-2 B domain antagonizes vascular endothelial growth factor bioactivity and inhibits tumor progression. Molecular Cancer Research, 8(8), 1063–1073. https://doi.org/10.1158/1541-7786.MCR-10-0157
Immormino, R. M., Lauzier, D. C., Nakano, H., Hernandez, M. L., Alexis, N. E., Ghio, A. J., Tilley, S. L., Doerschuk, C. M., Peden, D. B., Cook, D. N., & Moran, T. P. (2018). Neuropilin-2 regulates airway inflammatory responses to inhaled lipopolysaccharide. American Journal of Physiology. Lung Cellular and Molecular Physiology, 315(2), L202–L211. https://doi.org/10.1152/ajplung.00067.2018
Chen, H., He, Z., Bagri, A., & Tessier-Lavigne, M. (1998). Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron, 21(6), 1283–1290. https://doi.org/10.1016/s0896-6273(00)80648-0
Yuan, L., Moyon, D., Pardanaud, L., Breant, C., Karkkainen, M. J., Alitalo, K., & Eichmann, A. (2002). Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development, 129(20), 4797–4806.
Giger, R. J., Cloutier, J. F., Sahay, A., Prinjha, R. K., Levengood, D. V., Moore, S. E., Pickering, S., Simmons, D., Rastan, S., Walsh, F. S., Kolodkin, A. L., Ginty, D. D., & Geppert, M. (2000). Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron, 25(1), 29–41. https://doi.org/10.1016/s0896-6273(00)80869-7
Neufeld, G., Cohen, T., Shraga, N., Lange, T., Kessler, O., & Herzog, Y. (2002). The neuropilins: Multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends in Cardiovascular Medicine, 12(1), 13–19. https://doi.org/10.1016/s1050-1738(01)00140-2
Sahay, A., Molliver, M. E., Ginty, D. D., & Kolodkin, A. L. (2003). Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. Journal of Neuroscience, 23(17), 6671–6680.
Lumb, R., Wiszniak, S., Kabbara, S., Scherer, M., Harvey, N., & Schwarz, Q. (2014). Neuropilins define distinct populations of neural crest cells. Neural Development, 9, 24. https://doi.org/10.1186/1749-8104-9-24
Shiflett, M. W., Gavin, M., & Tran, T. S. (2015). Altered hippocampal-dependent memory and motor function in neuropilin 2-deficient mice. Translational Psychiatry, 5, e521. https://doi.org/10.1038/tp.2015.17
Siemerink, M. J., Klaassen, I., Vogels, I. M., Griffioen, A. W., Van Noorden, C. J., & Schlingemann, R. O. (2012). CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis, 15(1), 151–163. https://doi.org/10.1007/s10456-011-9251-z
Coma, S., Allard-Ratick, M., Akino, T., van Meeteren, L. A., Mammoto, A., & Klagsbrun, M. (2013). GATA2 and Lmo2 control angiogenesis and lymphangiogenesis via direct transcriptional regulation of neuropilin-2. Angiogenesis, 16(4), 939–952. https://doi.org/10.1007/s10456-013-9370-9
German, A. E., Mammoto, T., Jiang, E., Ingber, D. E., & Mammoto, A. (2014). Paxillin controls endothelial cell migration and tumor angiogenesis by altering neuropilin 2 expression. Journal of Cell Science, 127(Pt 8), 1672–1683. https://doi.org/10.1242/jcs.132316
Fujita, H., Zhang, B., Sato, K., Tanaka, J., & Sakanaka, M. (2001). Expressions of neuropilin-1, neuropilin-2 and semaphorin 3A mRNA in the rat brain after middle cerebral artery occlusion. Brain Research, 914(1–2), 1–14. https://doi.org/10.1016/s0006-8993(01)02765-2
Dallinga, M. G., Habani, Y. I., Schimmel, A. W. M., Dallinga-Thie, G. M., van Noorden, C. J. F., Klaassen, I., Schlingemann, R. O. (2021). The role of heparan sulfate and neuropilin 2 in VEGFA signaling in human endothelial tip cells and non-tip cells during angiogenesis in vitro. Cells. 10(4). https://doi.org/10.3390/cells10040926.
Soker, S., Takashima, S., Miao, H. Q., Neufeld, G., & Klagsbrun, M. (1998). Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell., 92(6), 735–45. https://doi.org/10.1016/s0092-8674(00)81402-6
Hong, Y. K., Harvey, N., Noh, Y. H., Schacht, V., Hirakawa, S., Detmar, M., & Oliver, G. (2002). Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Developmental Dynamics, 225(3), 351–357. https://doi.org/10.1002/dvdy.10163
Cohen, T., Herzog, Y., Brodzky, A., Greenson, J. K., Eldar, S., Gluzman-Poltorak, Z., Neufeld, G., & Resnick, M. B. (2002). Neuropilin-2 is a novel marker expressed in pancreatic islet cells and endocrine pancreatic tumours. The Journal of Pathology, 198(1), 77–82. https://doi.org/10.1002/path.1179
Cheppudira, B. P., Girard, B. M., Malley, S. E., Schutz, K. C., May, V., & Vizzard, M. A. (2008). Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. American Journal of Physiology. Renal Physiology, 295(3), F826–F836. https://doi.org/10.1152/ajprenal.90305.2008
Saban, M. R., Sferra, T. J., Davis, C. A., Simpson, C., Allen, A., Maier, J., Fowler, B., Knowlton, N., Birder, L., Wu, X. R., & Saban, R. (2010). Neuropilin-VEGF signaling pathway acts as a key modulator of vascular, lymphatic, and inflammatory cell responses of the bladder to intravesical BCG treatment. American Journal of Physiology Renal Physiology, 299(6), F1245–F1256. https://doi.org/10.1152/ajprenal.00352.2010
Man, X. Y., Yang, X. H., Cai, S. Q., Yao, Y. G., & Zheng, M. (2006). Immunolocalization and expression of vascular endothelial growth factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes in human epidermis. Molecular Medicine, 12(7–8), 127–136. https://doi.org/10.2119/2006-00024.Man
Drenberg, C. D., Livingston, S., Chen, R., Kruk, P. A., & Nicosia, S. V. (2009). Expression of semaphorin 3F and its receptors in epithelial ovarian cancer, fallopian tubes, and secondary mullerian tissues. Obstetrics and Gynecology International, 2009, 730739. https://doi.org/10.1155/2009/730739
Cohen, T., Gluzman-Poltorak, Z., Brodzky, A., Meytal, V., Sabo, E., Misselevich, I., Hassoun, M., Boss, J. H., Resnick, M., Shneyvas, D., Eldar, S., & Neufeld, G. (2001). Neuroendocrine cells along the digestive tract express neuropilin-2. Biochemical and Biophysical Research Communications, 284(2), 395–403. https://doi.org/10.1006/bbrc.2001.4958
Hansel, D. E., Wilentz, R. E., Yeo, C. J., Schulick, R. D., Montgomery, E., & Maitra, A. (2004). Expression of neuropilin-1 in high-grade dysplasia, invasive cancer, and metastases of the human gastrointestinal tract. American Journal of Surgical Pathology, 28(3), 347–356. https://doi.org/10.1097/00000478-200403000-00007
Jubb, A. M., Sa, S. M., Ratti, N., Strickland, L. A., Schmidt, M., Callahan, C. A., & Koeppen, H. (2012). Neuropilin-2 expression in cancer. Histopathology, 61(3), 340–349. https://doi.org/10.1111/j.1365-2559.2012.04224.x
Verlinden, L., Kriebitzsch, C., Beullens, I., Tan, B. K., Carmeliet, G., & Verstuyf, A. (2013). Nrp2 deficiency leads to trabecular bone loss and is accompanied by enhanced osteoclast and reduced osteoblast numbers. Bone, 55(2), 465–475. https://doi.org/10.1016/j.bone.2013.03.023
Borkowetz, A., Froehner, M., Rauner, M., Conrad, S., Erdmann, K., Mayr, T., Datta, K., Hofbauer, L. C., Baretton, G. B., Wirth, M., Fuessel, S., Toma, M., & Muders, M. H. (2020). Neuropilin-2 is an independent prognostic factor for shorter cancer-specific survival in patients with acinar adenocarcinoma of the prostate. International Journal of Cancer, 146(9), 2619–2627. https://doi.org/10.1002/ijc.32679
Handa, A., Tokunaga, T., Tsuchida, T., Lee, Y. H., Kijima, H., Yamazaki, H., Ueyama, Y., Fukuda, H., & Nakamura, M. (2000). Neuropilin-2 expression affects the increased vascularization and is a prognostic factor in osteosarcoma. International Journal of Oncology, 17(2), 291–295. https://doi.org/10.3892/ijo.17.2.291
Kawakami, T., Tokunaga, T., Hatanaka, H., Kijima, H., Yamazaki, H., Abe, Y., Osamura, Y., Inoue, H., Ueyama, Y., & Nakamura, M. (2002). Neuropilin 1 and neuropilin 2 co-expression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer, 95(10), 2196–2201. https://doi.org/10.1002/cncr.10936
Osada, R., Horiuchi, A., Kikuchi, N., Ohira, S., Ota, M., Katsuyama, Y., & Konishi, I. (2006). Expression of semaphorins, vascular endothelial growth factor, and their common receptor neuropilins and alleic loss of semaphorin locus in epithelial ovarian neoplasms: Increased ratio of vascular endothelial growth factor to semaphorin is a poor prognostic factor in ovarian carcinomas. Human Pathology, 37(11), 1414–1425. https://doi.org/10.1016/j.humpath.2006.04.031
Yasuoka, H., Kodama, R., Tsujimoto, M., Yoshidome, K., Akamatsu, H., Nakahara, M., Inagaki, M., Sanke, T., & Nakamura, Y. (2009). Neuropilin-2 expression in breast cancer: Correlation with lymph node metastasis, poor prognosis, and regulation of CXCR4 expression. BMC Cancer, 9, 220. https://doi.org/10.1186/1471-2407-9-220
Fakhari, M., Pullirsch, D., Abraham, D., Paya, K., Hofbauer, R., Holzfeind, P., Hofmann, M., & Aharinejad, S. (2002). Selective upregulation of vascular endothelial growth factor receptors neuropilin-1 and -2 in human neuroblastoma. Cancer, 94(1), 258–263. https://doi.org/10.1002/cncr.10177
Wittmann, P., Grubinger, M., Groger, C., Huber, H., Sieghart, W., Peck-Radosavljevic, M., & Mikulits, W. (2015). Neuropilin-2 induced by transforming growth factor-beta augments migration of hepatocellular carcinoma cells. BMC Cancer, 15, 909. https://doi.org/10.1186/s12885-015-1919-0
Evanno, E., Godet, J., Piccirilli, N., Guilhot, J., Milin, S., Gombert, J. M., Fouchaq, B., & Roche, J. (2017). Tri-methylation of H3K79 is decreased in TGF-beta1-induced epithelial-to-mesenchymal transition in lung cancer. Clinical Epigenetics, 9, 80. https://doi.org/10.1186/s13148-017-0380-0
Gemmill, R. M., Nasarre, P., Nair-Menon, J., Cappuzzo, F., Landi, L., D'Incecco, A., Uramoto, H., Yoshida, T., Haura, E.B., Armeson, K., Drabkin, H. A. (2017). The neuropilin 2 isoform NRP2b uniquely supports TGFbeta-mediated progression in lung cancer. Science Signaling, 10(462). https://doi.org/10.1126/scisignal.aag0528.
Zhao, M., Zhang, M., Tao, Z., Cao, J., Wang, L., & Hu, X. (2020). miR-331-3p Suppresses cell proliferation in TNBC cells by downregulating NRP2. Technology in Cancer Research & Treatment, 19, 1533033820905824. https://doi.org/10.1177/1533033820905824
Epis, M. R., Giles, K. M., Candy, P. A., Webster, R. J., & Leedman, P. J. (2014). miR-331-3p regulates expression of neuropilin-2 in glioblastoma. Journal of Neuro-oncology, 116(1), 67–75. https://doi.org/10.1007/s11060-013-1271-7
Do, Y., Cho, J. G., Park, J. Y., Oh, S., Park, D., Yoo, K. H., Lee, M. S., Kwon, B. S., Kim, J., Yang, Y. (2020). MiR-146a Regulates migration and invasion by targeting NRP2 in circulating-tumor cell mimicking suspension cells. Genes (Basel), 12(1). https://doi.org/10.3390/genes12010045.
Chen, Y., Huang, S., Wu, B., Fang, J., Zhu, M., Sun, L., Zhang, L., Zhang, Y., Sun, M., Guo, L., & Wang, S. (2017). Transforming growth factor-beta1 promotes breast cancer metastasis by downregulating miR-196a-3p expression. Oncotarget, 8(30), 49110–49122. https://doi.org/10.18632/oncotarget.16308
Liu, C., Li, M., Hu, Y., Shi, N., Yu, H., Liu, H., & Lian, H. (2016). miR-486-5p attenuates tumor growth and lymphangiogenesis by targeting neuropilin-2 in colorectal carcinoma. Oncotargets and Therapy, 9, 2865–2871. https://doi.org/10.2147/OTT.S103460
Liu, A., Liu, L., & Lu, H. (2019). LncRNA XIST facilitates proliferation and epithelial-mesenchymal transition of colorectal cancer cells through targeting miR-486-5p and promoting neuropilin-2. Journal of Cellular Physiology, 234(8), 13747–13761. https://doi.org/10.1002/jcp.28054
Wang, Y., Yin, H., & Chen, X. (2021). Circ-LDLRAD3 Enhances cell growth, migration, and invasion and inhibits apoptosis by regulating MiR-224-5p/NRP2 axis in gastric cancer. Digestive Diseases and Sciences, 66(11), 3862–3871. https://doi.org/10.1007/s10620-020-06733-1
Cobos, I., Borello, U., & Rubenstein, J. L. (2007). Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron, 54(6), 873–888. https://doi.org/10.1016/j.neuron.2007.05.024
Yilmaz, M., Maass, D., Tiwari, N., Waldmeier, L., Schmidt, P., Lehembre, F., & Christofori, G. (2011). Transcription factor Dlx2 protects from TGFbeta-induced cell-cycle arrest and apoptosis. EMBO Journal, 30(21), 4489–4499. https://doi.org/10.1038/emboj.2011.319
Ghanem, N., Andrusiak, M. G., Svoboda, D., Al Lafi, S. M., Julian, L. M., McClellan, K. A., De Repentigny, Y., Kothary, R., Ekker, M., Blais, A., Park, D. S., & Slack, R. S. (2012). The Rb/E2F pathway modulates neurogenesis through direct regulation of the Dlx1/Dlx2 bigene cluster. Journal of Neuroscience, 32(24), 8219–8230. https://doi.org/10.1523/JNEUROSCI.1344-12.2012
Park, D. H., Hong, S. J., Salinas, R. D., Liu, S. J., Sun, S. W., Sgualdino, J., Testa, G., Matzuk, M. M., Iwamori, N., & Lim, D. A. (2014). Activation of neuronal gene expression by the JMJD3 demethylase is required for postnatal and adult brain neurogenesis. Cell Reports, 8(5), 1290–1299. https://doi.org/10.1016/j.celrep.2014.07.060
Morini, M., Astigiano, S., Gitton, Y., Emionite, L., Mirisola, V., Levi, G., & Barbieri, O. (2010). Mutually exclusive expression of DLX2 and DLX5/6 is associated with the metastatic potential of the human breast cancer cell line MDA-MB-231. BMC Cancer, 10, 649. https://doi.org/10.1186/1471-2407-10-649
Samuel, S., & Naora, H. (2005). Homeobox gene expression in cancer: Insights from developmental regulation and deregulation. European Journal of Cancer, 41(16), 2428–2437. https://doi.org/10.1016/j.ejca.2005.08.014
Ferrari, N., Palmisano, G. L., Paleari, L., Basso, G., Mangioni, M., Fidanza, V., Albini, A., Croce, C. M., Levi, G., & Brigati, C. (2003). DLX genes as targets of ALL-1: DLX 2,3,4 down-regulation in t(4;11) acute lymphoblastic leukemias. Journal of Leukocyte Biology, 74(2), 302–305. https://doi.org/10.1189/jlb.1102581
Lv, T., Wu, X., Sun, L., Hu, Q., Wan, Y., Wang, L., Zhao, Z., Tu, X., & Xiao, Z. J. (2017). p53–R273H upregulates neuropilin-2 to promote cell mobility and tumor metastasis. Cell Death & Disease, 8(8), e2995. https://doi.org/10.1038/cddis.2017.376
Ji, T., Guo, Y., Kim, K., McQueen, P., Ghaffar, S., Christ, A., Lin, C., Eskander, R., Zi, X., & Hoang, B. H. (2015). Neuropilin-2 expression is inhibited by secreted Wnt antagonists and its down-regulation is associated with reduced tumor growth and metastasis in osteosarcoma. Molecular Cancer, 14, 86. https://doi.org/10.1186/s12943-015-0359-4
Lucci, V., Di Palma, T., & Zannini, M. (2015). Neuropilin-2 Is a newly identified target of PAX8 in thyroid cells. PLoS One, 10(6), e0128315. https://doi.org/10.1371/journal.pone.0128315
Muders, M. H., Zhang, H., Wang, E., Tindall, D. J., & Datta, K. (2009). Vascular endothelial growth factor-C protects prostate cancer cells from oxidative stress by the activation of mammalian target of rapamycin complex-2 and AKT-1. Cancer Research, 69(15), 6042–6048. https://doi.org/10.1158/0008-5472.CAN-09-0552
Dutta, S., Roy, S., Polavaram, N. S., Stanton, M. J., Zhang, H., Bhola, T., Honscheid, P., Donohue, T. M., Jr., Band, H., Batra, S. K., Muders, M. H., & Datta, K. (2016). Neuropilin-2 regulates endosome maturation and EGFR trafficking to support cancer cell pathobiology. Cancer Research, 76(2), 418–428. https://doi.org/10.1158/0008-5472.CAN-15-1488
Roy, S., Bag, A. K., Dutta, S., Polavaram, N. S., Islam, R., Schellenburg, S., Banwait, J., Guda, C., Ran, S., Hollingsworth, M. A., Singh, R. K., Talmadge, J. E., Muders, M. H., Batra, S. K., & Datta, K. (2018). Macrophage-derived neuropilin-2 exhibits novel tumor-promoting functions. Cancer Research, 78(19), 5600–5617. https://doi.org/10.1158/0008-5472.CAN-18-0562
Bagri, A., Tessier-Lavigne, M., & Watts, R. J. (2009). Neuropilins in tumor biology. Clinical Cancer Research, 15(6), 1860–1864.
Uniewicz, K. A., & Fernig, D. G. (2008). Neuropilins: A versatile partner of extracellular molecules that regulate development and disease. Frontiers in Bioscience, 13, 4339–4360. https://doi.org/10.2741/3008
Prud’homme, G. J., & Glinka, Y. (2012). Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget, 3(9), 921–939. https://doi.org/10.18632/oncotarget.626
Roche, J., Boldog, F., Robinson, M., Robinson, L., Varella-Garcia, M., Swanton, M., Waggoner, B., Fishel, R., Franklin, W., Gemmill, R., & Drabkin, H. (1996). Distinct 3p21.3 deletions in lung cancer and identification of a new human semaphorin. Oncogene., 12(6), 1289–97.
Joseph, D., Ho, S. M., & Syed, V. (2010). Hormonal regulation and distinct functions of semaphorin-3B and semaphorin-3F in ovarian cancer. Molecular Cancer Therapeutics, 9(2), 499–509. https://doi.org/10.1158/1535-7163.MCT-09-0664
Chabbert-de Ponnat, I., Buffard, V., Leroy, K., Bagot, M., Bensussan, A., Wolkenstein, P., & Marie-Cardine, A. (2006). Antiproliferative effect of semaphorin 3F on human melanoma cell lines. The Journal of Investigative Dermatology, 126(10), 2343–2345. https://doi.org/10.1038/sj.jid.5700382
Nasarre, P., Kusy, S., Constantin, B., Castellani, V., Drabkin, H. A., Bagnard, D., & Roche, J. (2005). Semaphorin SEMA3F has a repulsing activity on breast cancer cells and inhibits E-cadherin-mediated cell adhesion. Neoplasia, 7(2), 180–189. https://doi.org/10.1593/neo.04481
Neufeld, G., & Kessler, O. (2008). The semaphorins: Versatile regulators of tumour progression and tumour angiogenesis. Nature Reviews Cancer, 8(8), 632–645. https://doi.org/10.1038/nrc2404
Potiron, V. A., Sharma, G., Nasarre, P., Clarhaut, J. A., Augustin, H. G., Gemmill, R. M., Roche, J., & Drabkin, H. A. (2007). Semaphorin SEMA3F affects multiple signaling pathways in lung cancer cells. Cancer Research, 67(18), 8708–8715. https://doi.org/10.1158/0008-5472.CAN-06-3612
Sulpice, E., Plouet, J., Berge, M., Allanic, D., Tobelem, G., & Merkulova-Rainon, T. (2008). Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood, 111(4), 2036–2045. https://doi.org/10.1182/blood-2007-04-084269
Matsushita, A., Gotze, T., & Korc, M. (2007). Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Research, 67(21), 10309–10316. https://doi.org/10.1158/0008-5472.CAN-07-3256
Hu, B., Guo, P., Bar-Joseph, I., Imanishi, Y., Jarzynka, M. J., Bogler, O., Mikkelsen, T., Hirose, T., Nishikawa, R., & Cheng, S. Y. (2007). Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene, 26(38), 5577–5586. https://doi.org/10.1038/sj.onc.1210348
Pellet-Many, C., Mehta, V., Fields, L., Mahmoud, M., Lowe, V., Evans, I., Ruivo, J., & Zachary, I. (2015). Neuropilins 1 and 2 mediate neointimal hyperplasia and re-endothelialization following arterial injury. Cardiovascular Research, 108(2), 288–298. https://doi.org/10.1093/cvr/cvv229
Glinka, Y., Stoilova, S., Mohammed, N., & Prud’homme, G. J. (2011). Neuropilin-1 exerts co-receptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta. Carcinogenesis, 32(4), 613–621. https://doi.org/10.1093/carcin/bgq281
Young, G. D., & Murphy-Ullrich, J. E. (2004). Molecular interactions that confer latency to transforming growth factor-beta. Journal of Biological Chemistry, 279(36), 38032–38039. https://doi.org/10.1074/jbc.M405658200
Grandclement, C., Pallandre, J. R., Valmary Degano, S., Viel, E., Bouard, A., Balland, J., Remy-Martin, J. P., Simon, B., Rouleau, A., Boireau, W., Klagsbrun, M., Ferrand, C., & Borg, C. (2011). Neuropilin-2 expression promotes TGF-beta1-mediated epithelial to mesenchymal transition in colorectal cancer cells. PLoS One, 6(7), e20444. https://doi.org/10.1371/journal.pone.0020444
Guarino, M. (2007). Epithelial-mesenchymal transition and tumour invasion. International Journal of Biochemistry & Cell Biology, 39(12), 2153–2160. https://doi.org/10.1016/j.biocel.2007.07.011
Samuel, S., Gaur, P., Fan, F., Xia, L., Gray, M. J., Dallas, N. A., Bose, D., Rodriguez-Aguayo, C., Lopez-Berestein, G., Plowman, G., Bagri, A., Sood, A. K., & Ellis, L. M. (2011). Neuropilin-2 mediated beta-catenin signaling and survival in human gastro-intestinal cancer cell lines. PLoS One, 6(10), e23208. https://doi.org/10.1371/journal.pone.0023208
Gray, M. J., Van Buren, G., Dallas, N. A., Xia, L., Wang, X., Yang, A. D., Somcio, R. J., Lin, Y. G., Lim, S., Fan, F., Mangala, L. S., Arumugam, T., Logsdon, C. D., Lopez-Berestein, G., Sood, A. K., & Ellis, L. M. (2008). Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. Journal of the National Cancer Institute, 100(2), 109–120. https://doi.org/10.1093/jnci/djm279
Dallas, N. A., Gray, M. J., Xia, L., Fan, F., van Buren, G., II., Gaur, P., Samuel, S., Lim, S. J., Arumugam, T., Ramachandran, V., Wang, H., & Ellis, L. M. (2008). Neuropilin-2-mediated tumor growth and angiogenesis in pancreatic adenocarcinoma. Clin Cancer Res., 14(24), 8052–60. https://doi.org/10.1158/1078-0432.CCR-08-1520
Kim, W. H., Lee, S. H., Jung, M. H., Seo, J. H., Kim, J., Kim, M. A., & Lee, Y. M. (2009). Neuropilin2 expressed in gastric cancer endothelial cells increases the proliferation and migration of endothelial cells in response to VEGF. Experimental Cell Research, 315(13), 2154–2164. https://doi.org/10.1016/j.yexcr.2009.04.018
Alghamdi, A. A. A., Benwell, C. J., Atkinson, S. J., Lambert, J., Johnson, R. T., & Robinson, S. D. (2020). NRP2 as an emerging angiogenic player; promoting endothelial cell adhesion and migration by regulating recycling of alpha5 integrin. Frontiers in Cell and Development Biology, 8, 395. https://doi.org/10.3389/fcell.2020.00395
Roy, S., Bag, A. K., Singh, R. K., Talmadge, J. E., Batra, S. K., & Datta, K. (2017). Multifaceted role of neuropilins in the immune system: Potential targets for immunotherapy. Frontiers in Immunology, 8, 1228. https://doi.org/10.3389/fimmu.2017.01228
Goel, H. L., Chang, C., Pursell, B., Leav, I., Lyle, S., Xi, H. S., Hsieh, C. C., Adisetiyo, H., Roy-Burman, P., Coleman, I. M., Nelson, P. S., Vessella, R. L., Davis, R. J., Plymate, S. R., & Mercurio, A. M. (2012). VEGF/neuropilin-2 regulation of Bmi-1 and consequent repression of IGF-IR define a novel mechanism of aggressive prostate cancer. Cancer Discovery, 2(10), 906–921. https://doi.org/10.1158/2159-8290.CD-12-0085
Polavaram, N. S., Dutta, S., Islam, R., Bag, A. K., Roy, S., Poitz, D., Karnes, J., Hofbauer, L. C., Kohli, M., Costello, B. A., Jimenez, R., Batra, S. K., Teply, B. A., Muders, M. H., & Datta, K. (2021). Tumor- and osteoclast-derived NRP2 in prostate cancer bone metastases. Bone Research, 9(1), 24. https://doi.org/10.1038/s41413-021-00136-2
Halabi, S., Kelly, W. K., Ma, H., Zhou, H., Solomon, N. C., Fizazi, K., Tangen, C. M., Rosenthal, M., Petrylak, D. P., Hussain, M., Vogelzang, N. J., Thompson, I. M., Chi, K. N., de Bono, J., Armstrong, A. J., Eisenberger, M. A., Fandi, A., Li, S., Araujo, J. C., … Small, E. J. (2016). Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. Journal of Clinical Oncology, 34(14), 1652–1659. https://doi.org/10.1200/JCO.2015.65.7270
Abida, W., Cyrta, J., Heller, G., Prandi, D., Armenia, J., Coleman, I., Cieslik, M., Benelli, M., Robinson, D., Van Allen, E. M., Sboner, A., Fedrizzi, T., Mosquera, J. M., Robinson, B. D., De Sarkar, N., Kunju, L. P., Tomlins, S., Wu, Y. M., Nava Rodrigues, D., … Sawyers, C. L. (2019). Genomic correlates of clinical outcome in advanced prostate cancer. Proceedings of the National Academy of Sciences of the United States of America, 116(23), 11428–11436. https://doi.org/10.1073/pnas.1902651116
Stanton, M. J., Dutta, S., Zhang, H., Polavaram, N. S., Leontovich, A. A., Honscheid, P., Sinicrope, F. A., Tindall, D. J., Muders, M. H., & Datta, K. (2013). Autophagy control by the VEGF-C/NRP-2 axis in cancer and its implication for treatment resistance. Cancer Research, 73(1), 160–171. https://doi.org/10.1158/0008-5472.CAN-11-3635
Dutta, S., Roy, S., Polavaram, N. S., Baretton, G. B., Muders, M. H., Batra, S., & Datta, K. (2016). NRP2 transcriptionally regulates its downstream effector WDFY1. Science and Reports, 6, 23588. https://doi.org/10.1038/srep23588
Dutta, S. P. N., Islam, R., Bhattacharya, S., Bodas, S., Mayr, T., Roy, S., Albala, S. Y., Toma, M. I., Darehshouri, A., Borkowetz, A., Conrad, S., Fuessel, S., Wirth, M., Baretton, G., Hofbauer, L., Ghosh, P., Pienta, K. J., Klinkebiel, D., Batra, S. K., Muders, M. H., Datta, K. (2022). Neuropilin-2 regulates androgen-receptor transcriptional activity in advanced prostate cancer. Oncogene.
Goel, H. L., Pursell, B., Shultz, L. D., Greiner, D. L., Brekken, R. A., Vander Kooi, C. W., & Mercurio, A. M. (2016). P-Rex1 promotes resistance to VEGF/VEGFR-targeted therapy in prostate cancer. Cell Reports, 14(9), 2193–2208. https://doi.org/10.1016/j.celrep.2016.02.016
Islam, R. M., J. Polavaram, N. Bhattacharya, S. Hong, Z, Bodas, S. Sharma, S. Bouska, A. Gilbreath, T. Said, A. Smith, L. M. Teply, B. A. Muders, M. H. Batra, S. K. Datta, K. Dutta, S (2022). Neuropilin-2 axis in regulating secretory phenotype of neuroendocrine-like prostate cancer cells: Implication in therapy resistance. Cell Reports.
Keck, B., Wach, S., Taubert, H., Zeiler, S., Ott, O. J., Kunath, F., Hartmann, A., Bertz, S., Weiss, C., Honscheid, P., Schellenburg, S., Rodel, C., Baretton, G. B., Sauer, R., Fietkau, R., Wullich, B., Krause, F. S., Datta, K., & Muders, M. H. (2015). Neuropilin-2 and its ligand VEGF-C predict treatment response after transurethral resection and radiochemotherapy in bladder cancer patients. International Journal of Cancer, 136(2), 443–451. https://doi.org/10.1002/ijc.28987
Forster, S., Givehchi, M., Nitschke, K., Mayr, T., Kilian, K., Dutta, S., Datta, K., Nuhn, P., Popovic, Z., Muders, M. H., Erben, P. (2021). Neuropilin-2 and its transcript variants correlate with clinical outcome in bladder cancer. Genes (Basel), 12(4). https://doi.org/10.3390/genes12040550.
Deng, C., Guo, H., Yan, D., Liang, T., Ye, X., & Li, Z. (2021). Pancancer analysis of neurovascular-related NRP family genes as potential prognostic biomarkers of bladder urothelial carcinoma. BioMed Research International, 2021, 5546612. https://doi.org/10.1155/2021/5546612
Schulz, A., Gorodetska, I., Behrendt, R., Fuessel, S., Erdmann, K., Foerster, S., Datta, K., Mayr, T., Dubrovska, A., & Muders, M. H. (2019). Linking NRP2 With EMT and chemoradioresistance in bladder cancer. Frontiers in Oncology, 9, 1461. https://doi.org/10.3389/fonc.2019.01461
Cao, Y., Hoeppner, L. H., Bach, S., Guangqi, E., Guo, Y., Wang, E., Wu, J., Cowley, M. J., Chang, D. K., Waddell, N., Grimmond, S. M., Biankin, A. V., Daly, R. J., Zhang, X., & Mukhopadhyay, D. (2013). Neuropilin-2 promotes extravasation and metastasis by interacting with endothelial alpha5 integrin. Cancer Research, 73(14), 4579–90. https://doi.org/10.1158/0008-5472.CAN-13-0529
Cetin, B., Gonul, I. I., Buyukberber, S., Afsar, B., Gumusay, O., Algin, E., Turan, N., Ozet, A., Benekli, M., & Coskun, U. (2015). The impact of immunohistochemical staining with ezrin-carbonic anhydrase IX and neuropilin-2 on prognosis in patients with metastatic renal cell cancer receiving tyrosine kinase inhibitors. Tumour Biology, 36(11), 8471–8478. https://doi.org/10.1007/s13277-015-3589-6
Bollard, J., Patte, C., Radkova, K., Massoma, P., Chardon, L., Valantin, J., Gadot, N., Goddard, I., Vercherat, C., Hervieu, V., Gouysse, G., Poncet, G., Scoazec, J. Y., Walter, T., & Roche, C. (2019). Neuropilin-2 contributes to tumor progression in preclinical models of small intestinal neuroendocrine tumors. The Journal of Pathology, 249(3), 343–355. https://doi.org/10.1002/path.5321
Dong, X., Guo, W., Zhang, S., Wu, T., Sun, Z., Yan, S., & Zheng, S. (2017). Elevated expression of neuropilin-2 associated with unfavorable prognosis in hepatocellular carcinoma. Oncotargets and Therapy, 10, 3827–3833. https://doi.org/10.2147/OTT.S139044
Eloranta, K., Nousiainen, R., Cairo, S., Pakarinen, M. P., Wilson, D. B., Pihlajoki, M., & Heikinheimo, M. (2021). Neuropilin-2 is associated with increased hepatoblastoma cell viability and motility. Frontiers in Pediatrics, 9, 660482. https://doi.org/10.3389/fped.2021.660482
Fukahi, K., Fukasawa, M., Neufeld, G., Itakura, J., & Korc, M. (2004). Aberrant expression of neuropilin-1 and -2 in human pancreatic cancer cells. Clinical Cancer Research, 10(2), 581–590. https://doi.org/10.1158/1078-0432.ccr-0930-03
Wang, L., Wang, L., Wang, S., Zhou, Z., Liu, Z., Xu, P., Luo, X., Wu, T., Luo, F., & Yan, J. (2021). N2E4, a Monoclonal antibody targeting neuropilin-2, inhibits tumor growth and metastasis in pancreatic ductal adenocarcinoma via suppressing FAK/Erk/HIF-1alpha signaling. Frontiers in Oncology, 11, 657008. https://doi.org/10.3389/fonc.2021.657008
Li, M., Zhang, Y., Feurino, L. W., Wang, H., Fisher, W. E., Brunicardi, F. C., Chen, C., & Yao, Q. (2008). Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Science, 99(4), 733–737. https://doi.org/10.1111/j.1349-7006.2008.00740.x
Marimuthu, S. L. I., Muniyan, S., Gautam, S., Nimmakayala, R. K., Rauth, S., Atri, P., Shah, A., Bhyravbhatla, N., Mallya, K., Grandgenett, P., Hollingsworth, M., Datta, K., Jain, M., Ponnusamy, M., and Batra, S. K. (2022). MUC16 promotes liver metastasis of pancreatic ductal adenocarcinoma by upregulating NRP2 associated cell adhesion molecular cancer research.
Wu, F., Zhou, Q., Yang, J., Duan, G. J., Ou, J. J., Zhang, R., Pan, F., Peng, Q. P., Tan, H., Ping, Y. F., Cui, Y. H., Qian, C., Yan, X. C., & Bian, X. W. (2011). Endogenous axon guiding chemorepulsant semaphorin-3F inhibits the growth and metastasis of colorectal carcinoma. Clinical Cancer Research, 17(9), 2702–2711. https://doi.org/10.1158/1078-0432.CCR-10-0839
Zhao, H., Hou, C., Hou, A., & Zhu, D. (2016). Concurrent expression of VEGF-C and neuropilin-2 is correlated with poor prognosis in glioblastoma. Tohoku Journal of Experimental Medicine, 238(2), 85–91. https://doi.org/10.1620/tjem.238.85
Zheng, X., Chopp, M., Lu, Y., Buller, B., & Jiang, F. (2013). MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Letters, 329(2), 146–154. https://doi.org/10.1016/j.canlet.2012.10.026
Nasarre, C., Koncina, E., Labourdette, G., Cremel, G., Roussel, G., Aunis, D., & Bagnard, D. (2009). Neuropilin-2 acts as a modulator of Sema3A-dependent glioma cell migration. Cell Adhesion & Migration, 3(4), 383–9. https://doi.org/10.4161/cam.3.4.9934
Timoshenko, A. V., Rastogi, S., & Lala, P. K. (2007). Migration-promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. British Journal of Cancer, 97(8), 1090–1098. https://doi.org/10.1038/sj.bjc.6603993
Goel, H. L., Pursell, B., Chang, C., Shaw, L. M., Mao, J., Simin, K., Kumar, P., Vander Kooi, C. W., Shultz, L. D., Greiner, D. L., Norum, J. H., Toftgard, R., Kuperwasser, C., & Mercurio, A. M. (2013). GLI1 regulates a novel neuropilin-2/alpha6beta1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Molecular Medicine, 5(4), 488–508. https://doi.org/10.1002/emmm.201202078
Elaimy, A. L., Guru, S., Chang, C., Ou, J., Amante, J. J., Zhu, L. J., Goel, H. L., Mercurio, A. M. (2018). VEGF-neuropilin-2 signaling promotes stem-like traits in breast cancer cells by TAZ-mediated repression of the Rac GAP beta2-chimaerin. Science Signaling, 11(528). https://doi.org/10.1126/scisignal.aao6897.
Goel, H. L., Pursell, B., Standley, C., Fogarty, K., & Mercurio, A. M. (2012). Neuropilin-2 regulates alpha6beta1 integrin in the formation of focal adhesions and signaling. Journal of Cell Science, 125(Pt 2), 497–506. https://doi.org/10.1242/jcs.094433
Elaimy, A. L., Amante, J. J., Zhu, L. J., Wang, M., Walmsley, C. S., FitzGerald, T. J., Goel, H. L., & Mercurio, A. M. (2019). The VEGF receptor neuropilin 2 promotes homologous recombination by stimulating YAP/TAZ-mediated Rad51 expression. Proceedings of the National Academy of Sciences of the United States of America, 116(28), 14174–14180. https://doi.org/10.1073/pnas.1821194116
Rushing, E. C., Stine, M. J., Hahn, S. J., Shea, S., Eller, M. S., Naif, A., Khanna, S., Westra, W. H., Jungbluth, A. A., Busam, K. J., Mahalingam, M., & Alani, R. M. (2012). Neuropilin-2: A novel biomarker for malignant melanoma? Human Pathology, 43(3), 381–389. https://doi.org/10.1016/j.humpath.2011.05.008
Rossi, M., Tuck, J., Kim, O. J., Panova, I., Symanowski, J. T., Mahalingam, M., Riker, A. I., Alani, R. M., & Ryu, B. (2014). Neuropilin-2 gene expression correlates with malignant progression in cutaneous melanoma. British Journal of Dermatology, 171(2), 403–408. https://doi.org/10.1111/bjd.12801
Wititsuwannakul, J., Mason, A. R., Klump, V. R., & Lazova, R. (2013). Neuropilin-2 as a useful marker in the differentiation between Spitzoid malignant melanoma and Spitz nevus. Journal of the American Academy of Dermatology, 68(1), 129–137. https://doi.org/10.1016/j.jaad.2012.07.009
Stine, M. J., Wang, C. J., Moriarty, W. F., Ryu, B., Cheong, R., Westra, W. H., Levchenko, A., & Alani, R. M. (2011). Integration of genotypic and phenotypic screening reveals molecular mediators of melanoma-stromal interaction. Cancer Research, 71(7), 2433–2444. https://doi.org/10.1158/0008-5472.CAN-10-1875
Moriarty, W. F., Kim, E., Gerber, S. A., Hammers, H., & Alani, R. M. (2016). Neuropilin-2 promotes melanoma growth and progression in vivo. Melanoma Research, 26(4), 321–328. https://doi.org/10.1097/CMR.0000000000000190
Vales, A., Kondo, R., Aichberger, K. J., Mayerhofer, M., Kainz, B., Sperr, W. R., Sillaber, C., Jager, U., & Valent, P. (2007). Myeloid leukemias express a broad spectrum of VEGF receptors including neuropilin-1 (NRP-1) and NRP-2. Leukaemia & Lymphoma, 48(10), 1997–2007. https://doi.org/10.1080/10428190701534424
Mendes-da-Cruz, D. A., Brignier, A. C., Asnafi, V., Baleydier, F., Messias, C. V., Lepelletier, Y., Bedjaoui, N., Renand, A., Smaniotto, S., Canioni, D., Milpied, P., Balabanian, K., Bousso, P., Lepretre, S., Bertrand, Y., Dombret, H., Ifrah, N., Dardenne, M., Macintyre, E., … Hermine, O. (2014). Semaphorin 3F and neuropilin-2 control the migration of human T-cell precursors. PLoS One, 9(7), e103405. https://doi.org/10.1371/journal.pone.0103405
Lee, G., Kang, Y. E., Oh, C., Liu, L., Jin, Y., Lim, M. A., Won, H. R., Chang, J. W., & Koo, B. S. (2020). Neuropilin-2 promotes growth and progression of papillary thyroid cancer cells. Auris, Nasus, Larynx, 47(5), 870–880. https://doi.org/10.1016/j.anl.2020.03.013
Finley, D. J., Arora, N., Zhu, B., Gallagher, L., & Fahey, T. J., 3rd. (2004). Molecular profiling distinguishes papillary carcinoma from benign thyroid nodules. Journal of Clinical Endocrinology and Metabolism, 89(7), 3214–3223. https://doi.org/10.1210/jc.2003-031811
Tu, D. G., Chang, W. W., Jan, M. S., Tu, C. W., Lu, Y. C., & Tai, C. K. (2016). Promotion of metastasis of thyroid cancer cells via NRP-2-mediated induction. Oncology Letters, 12(5), 4224–4230. https://doi.org/10.3892/ol.2016.5153
Yasuoka, H., Kodama, R., Hirokawa, M., Takamura, Y., Miyauchi, A., Inagaki, M., Sanke, T., & Nakamura, Y. (2011). Neuropilin-2 expression in papillary thyroid carcinoma: Correlation with VEGF-D expression, lymph node metastasis, and VEGF-D-induced aggressive cancer cell phenotype. Journal of Clinical Endocrinology and Metabolism, 96(11), E1857–E1861. https://doi.org/10.1210/jc.2011-1180
Lantuejoul, S., Constantin, B., Drabkin, H., Brambilla, C., Roche, J., & Brambilla, E. (2003). Expression of VEGF, semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. The Journal of Pathology, 200(3), 336–347. https://doi.org/10.1002/path.1367
Castro-Rivera, E., Ran, S., Thorpe, P., & Minna, J. D. (2004). Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proceedings of the National Academy of Sciences of the United States of America, 101(31), 11432–11437. https://doi.org/10.1073/pnas.0403969101
Nasarre, P., Gemmill, R. M., Potiron, V. A., Roche, J., Lu, X., Baron, A. E., Korch, C., Garrett-Mayer, E., Lagana, A., Howe, P. H., & Drabkin, H. A. (2013). Neuropilin-2 is upregulated in lung cancer cells during TGF-beta1-induced epithelial-mesenchymal transition. Cancer Research, 73(23), 7111–7121. https://doi.org/10.1158/0008-5472.CAN-13-1755
Wang, F. Q., Barfield, E., Dutta, S., Pua, T., & Fishman, D. A. (2009). VEGFR-2 silencing by small interference RNA (siRNA) suppresses LPA-induced epithelial ovarian cancer (EOC) invasion. Gynecologic Oncology, 115(3), 414–423. https://doi.org/10.1016/j.ygyno.2009.08.019
Kramer, A., Green, J., Pollard, J., Jr., & Tugendreich, S. (2014). Causal analysis approaches in ingenuity pathway analysis. Bioinformatics, 30(4), 523–530. https://doi.org/10.1093/bioinformatics/btt703
Linneberg, C., Toft, C. L. F., Kjaer-Sorensen, K., & Laursen, L. S. (2019). L1cam-mediated developmental processes of the nervous system are differentially regulated by proteolytic processing. Science and Reports, 9(1), 3716. https://doi.org/10.1038/s41598-019-39884-x
Maten, M. V., Reijnen, C., Pijnenborg, J. M. A., Zegers, M. M. (2019). L1 cell adhesion molecule in cancer, a systematic review on domain-specific functions. International Journal of Molecular Sciences 20(17). https://doi.org/10.3390/ijms20174180.
Sakurai, T. (2012). The role of NrCAM in neural development and disorders–Beyond a simple glue in the brain. Molecular and Cellular Neuroscience, 49(3), 351–363. https://doi.org/10.1016/j.mcn.2011.12.002
Wachowiak, R., Mayer, S., Suttkus, A., Martynov, I., Lacher, M., Melling, N., Izbicki, J. R., & Tachezy, M. (2019). CHL1 and NrCAM are primarily expressed in low grade pediatric neuroblastoma. Open Med (Wars)., 14, 920–927. https://doi.org/10.1515/med-2019-0109
Gorka, B., Skubis-Zegadlo, J., Mikula, M., Bardadin, K., Paliczka, E., & Czarnocka, B. (2007). NrCAM, a neuronal system cell-adhesion molecule, is induced in papillary thyroid carcinomas. British Journal of Cancer, 97(4), 531–538. https://doi.org/10.1038/sj.bjc.6603915
Ling, X. H., Fu, H., Chen, Z. Y., Lu, J. M., Zhuo, Y. J., Chen, J. H., Zhong, W. D., & Jia, Z. (2019). miR505 suppresses prostate cancer progression by targeting NRCAM. Oncology Reports, 42(3), 991–1004. https://doi.org/10.3892/or.2019.7231
Bendor, J. T., Logan, T. P., & Edwards, R. H. (2013). The function of alpha-synuclein. Neuron., 79(6), 1044–66. https://doi.org/10.1016/j.neuron.2013.09.004
Lerias, J. R., Pinto, M. C., Botelho, H. M., Awatade, N. T., Quaresma, M. C., Silva, I. A. L., Wanitchakool, P., Schreiber, R., Pepperkok, R., Kunzelmann, K., & Amaral, M. D. (2018). A novel microscopy-based assay identifies extended synaptotagmin-1 (ESYT1) as a positive regulator of anoctamin 1 traffic. Biochimica et Biophysica Acta, Molecular Cell Research, 1865(2), 421–431. https://doi.org/10.1016/j.bbamcr.2017.11.009
Rondas, D., Tomas, A., & Halban, P. A. (2011). Focal adhesion remodeling is crucial for glucose-stimulated insulin secretion and involves activation of focal adhesion kinase and paxillin. Diabetes, 60(4), 1146–1157. https://doi.org/10.2337/db10-0946
Dominiak, A., Chelstowska, B., Olejarz, W., Nowicka, G. (2020). Communication in the cancer microenvironment as a target for therapeutic interventions. Cancers (Basel), 12(5). https://doi.org/10.3390/cancers12051232.
Parker, M. W., & Vander Kooi, C. W. (2014). Microplate-based screening for small molecule inhibitors of neuropilin-2/vascular endothelial growth factor-C interactions. Analytical Biochemistry, 453, 4–6. https://doi.org/10.1016/j.ab.2014.02.017
Said, A. M., Parker, M. W., & Vander Kooi, C. W. (2020). Design, synthesis, and evaluation of a novel benzamidine-based inhibitor of VEGF-C binding to neuropilin-2. Bioorganic Chemistry, 100, 103856. https://doi.org/10.1016/j.bioorg.2020.103856
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Islam, R., Mishra, J., Bodas, S. et al. Role of Neuropilin-2-mediated signaling axis in cancer progression and therapy resistance. Cancer Metastasis Rev 41, 771–787 (2022). https://doi.org/10.1007/s10555-022-10048-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10048-0