Abstract
Cancer, especially when it has metastasized to different locations in the body, is notoriously difficult to treat. Metastatic cancer accounts for most cancer deaths and thus remains an enormous challenge. During the metastasis process, cancer cells negotiate a series of steps termed the “metastatic cascadeˮ that offer potential for developing anti-metastatic therapy strategies. Currently available conventional treatment and diagnostic methods addressing metastasis come with their own pitfalls and roadblocks. In this contribution, we comprehensively discuss the potential improvements that nanotechnology-aided approaches are able to bring, either alone or in combination with the existing conventional techniques, to the identification and treatment of metastatic disease. We tie specific nanotechnology-aided strategies to the complex biology of the different steps of the metastatic cascade in order to open up new avenues for fine-tuned targeting and development of anti-metastatic agents designed specifically to prevent or mitigate the metastatic outgrowth of cancer. We also present a viewpoint on the progress of translation of nanotechnology into cancer metastasis patient care.
Similar content being viewed by others
1 Introduction
Cancer metastasis is the spread of cancer cells from a primary tumor to the surrounding tissue or distant site to seed secondary/tertiary tumors. Cancer metastasis is responsible for considerable disability and more than 90% of cancer-associated mortality; nonetheless its exact mechanisms remain to be elucidated in depth. Metastasis represents one of the greatest challenges till date in cancer evaluation and treatment, not only because of the ability of metastatic cells to spread to different organs but also because of the consequent tumor cell heterogeneity, plasticity, and unique tumor microenvironment (TME) complexity that may respond differently to the treatment, thus underlying the greatest cause of failure of current metastatic cancer therapies [1, 2].
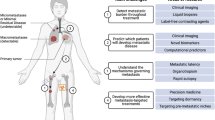
The metastatic process represents a complex multi-step and multi-directional succession of a series of cell-biological events termed the metastatic cascade. This cascade includes the loss of malignant cell adhesion at the primary tumor site conferring the cells the ability to detach, locally invade and enter the vasculature. As these cells are looking to home elsewhere, they intravasate and adhere to the vascular walls and disseminate through the circulatory system. They then squeeze through the endothelial barrier, extravasate at a distant site, and finally invade, form pre-metastatic niches, colonize, and proliferate at a new site to form micro- and macrometastatic tumor nodules or deposits (Fig. 1) [3,4,5]. During the process of the metastatic cascade, cancer cells go through several pathophysiological experiences such as epithelial to mesenchymal transition (EMT), wherein metastatic cells arising from either epithelial stem cells or differentiated epithelial cells transform into a tumor cell with mesenchymal features and evade immune attack [5,6,7]. As an alternative method, in case of peritoneal metastasis of abdominal cancers, malignant cells originating from primary abdominal organs spread through a transcoelomic mechanism as opposed to the hematogenous mechanism [8]. In either case, the cancer metastatic process is influenced by the primary tumor on site-specific metastasis (the “seed”), as well as the competence of the distal organ (the “soil”) [5, 9]. In other words, the most common sites for cancers to metastasize depend on the area the cancer starts in called the “primary site,ˮ the nearby vasculature, and the likelihood to spread to certain organs; and therefore, different tumors metastasize differently. Several biological mechanistic processes participate in this metastatic cascade, such as changes in cell polarity, remodeling of the cell cytoskeleton, acquired mutations, post-translational modifications and the expression of membrane proteins [10]. One of the most difficult aspects of diagnosing and treating metastatic cancer is that the cells often remain dormant and cannot be found until they emerge as incurable tumors, when it is already too late for successful treatment. Metastasis may have already initiated/occurred and advanced in number of patients even before they are diagnosed with primary cancer, and the metastatic process shows several variations depending on the cancer type. For example, breast cancer can remain latent for years and it is difficult to detect metastasis in such cancers, while lung cancer metastasis has often already formed and is detectable in multiple organs at the time of initial diagnosis. Also, in rare cases, patients have metastatic disease with no detectable primary tumor owing to a very small non-visible primary tumor also called as “cancer of unknown origin,ˮ which makes it even more difficult to treat. To add much more to this treatment complexity, some metastases possess features that are very diverse from a primary tumor, making it unrecognizable of its origin. For example, a large proportion of metastases of renal and breast cancer do not share common features with primary tumors [5, 11,12,13]. For a detailed description on metastasis, its pathophysiological characteristics, strategies and challenges to combat it, please refer to the following cited references [5,6,7].
Schematic representation of the metastatic cascade. Malignant cells detach from the primary tumor, traverse into the circulatory (including the lymphatic) systems, extravasate at a distant secondary site, invade and proliferate and colonize at a distant organ as micrometastasis and, finally, outgrow as macroscopic metastatic tumor deposits. Figure imported from Saxena and Cristofori [139]. Annotations on the figure represent references of nanotechnology-assisted targeting approaches addressing the various steps of the metastatic cascade as demonstrated in the figure and discussed in the text
A large spectrum of drugs that can be administered in cancer treatment are currently available towards addressing metastasis, but the main pitfalls are in the identification and analysis of the presence and extent of metastasis, and the appropriate targeting and drug delivery approaches. In majority of cases, primary and metastatic tumors are being treated with the same drugs as the primary cancer making the treatment complex and ineffective to combat established metastasis. Moreover, current conventional treatment/care strategies such as surgical removal, chemotherapy, immunotherapy, radiotherapy, and palliative care, all are accompanied by their own drawbacks and can be applied successfully to only a small number of patients diagnosed with metastatic cancer [6, 14]. Traditional chemotherapy and radiotherapy have disadvantages in efficacy and side effects because of unspecific distribution and indiscriminate cytotoxicity to cancer cells and normal cells. Current chemotherapy also faces problems such as lack of treatment specificity, heterogeneity, short half-life, poor solubility, occurrence of multi-drug resistance, and stem-like cells growth [15, 16]. All of the above treatment approaches emphasizes the need for solutions to prevent the systemic dissemination of malignant cells (metastatic disease) and secondary tumor formation and/or to reverse the fundamental metastatic processes, thus stressing the importance of the need of novel diagnostic/treatment approaches that can possibly reduce drawbacks and roadblocks of the conventional strategies.
Nanotechnology manipulates matter at the nanoscale to create structures, devices, and systems for medical and other uses. Nanoparticles (NPs) are the essential building blocks of nanotechnology that can potentially serve as targeting and/or detection agents owing to their unique physical and chemical features that allow tailored and tunable therapeutic and theranostic functions. Nanomaterial-based approaches and nanomedicine hold great promise and potential to improve anti-cancer therapy and evaluation. The advent of nanotechnology nanomedicines used in cancer therapy can possibly reduce the above-mentioned disadvantages and drawbacks of chemotherapy and other conventional therapeutic modalities [17, 18]. Traditionally, nanomedicines are used to modulate the biodistribution and the target site accumulation of systemically administered chemotherapeutic drugs by improving the latter’s specific and selective delivery, thereby improving the balance between their efficacy and toxicity. Further, biofunctionalized NPs loaded with drugs can be tailored to overcome biological barriers and to improve efficacy for the transport and cellular translocation of therapeutic molecules, while reducing morbidity [19]. A highly sensitive multi-modal nano-biocompound that has attached a delivery carrier with affinity for unique surface receptor proteins located inside the cellular wall, can deliver a desired active molecule in the desired tissue [20].
Several nanomedicine products have obtained regulatory approval, from the relevant regulatory authorities or agencies (in the USA and across the world), and have entered clinical practice while many others are under evaluation in preclinical/clinical settings to address a wide variety of indications in cancer and metastasis. For purpose of concise summary, Table 1 presents nano-formulated cancer medicines with granted regulatory approval, while Supplementary Material outlines current clinical studies investigating nanotechnology for cancer therapies and diagnostics. A perspective on the progress of translation of nanotechnology into patient care is provided further ahead in this review. Furthermore, directions that will fuel and foster the development of successful cancer (metastasis) nanomedicine therapies have also been discussed elsewhere. For more detailed information on the above-mentioned approved cancer nano-drugs, and future perspectives on investigational cancer nano-drugs, please refer to the following [13, 21,22,23,24,25].
It is believed that nanomedicines due to their multi-functionality and ability to deliver multiple drugs and even multiple treatment modalities in one construct could improve treatment of metastatic cancers and provide for its improved (early) detection and diagnosis. In this contribution, we will predominantly focus on discussing the utilization of nanotechnology-based therapeutics and diagnostics in combating metastatic cancers, and the added advantage nanotechnology brings to the conventional (or other) techniques/methods when used in combination (see Fig. 1 and Table 2). Majority of the discussion is dedicated to pre-clinical results. Our intention here is to signal the potential of nanotechnology in cancer metastasis, and simply most of the works to date are in pre-clinical stage. To put this in a proper context, one needs to remember that combating metastasis is a daunting problem and developing clinically worthy strategies is not easy. For instance, the inspection of iSearch–NIH grant database demonstrated that in the past 5 years, only about 10% of all cancer metastasis grants are focused on clinical efforts, while about 20% of all cancer grants are focused on clinical efforts. The translation of effective anti-metastatic treatments is slow across all treatment technologies and modalities, and this slow pace is not limited to nanotechnology.
Targeting tumor metastasis remains to date a critical challenge for tumor treatment, therefore the unique association of these nanotechnology advances with different targeted delivery strategies to counteract cancer metastasis, at the different metastatic cascade steps, is provided. This will enable the delineation of correlation between biology of metastasis, the appropriate design of nano approaches used, and the resulting prospects. Approaches towards overcoming the impending shortcomings and side effects of conventional techniques and nanotechnology is also incorporated into the discussed strategies. Finally, we will highlight the advancements, challenges, and perspectives of the combination efforts to combat the metastatic spread of malignant tumors. A viewpoint on the status of nanotechnology translation into patient care and the barriers that need to be overcome to accelerate translation of these technologies are also provided.
2 Nanoparticle-assisted targeting of the primary cancer to mitigate metastasis
A large number of studies have shown successful combating of tumor metastasis by NP-assisted targeting the primary cancer site towards several measures, such as inducing apoptosis of tumor cells; targeting the cancer stem cells (CSCs); hindering EMT; modulating the TME; or stimulating immune responses. For example, nanoliposomal formulation of ceramide, a bioactive sphingolipid, has shown favorable results in treating solid tumor metastasis in preclinical studies [13]. C6 ceramide-formulated liposomes significantly suppress cell proliferation and instigate apoptosis of highly aggressive metastatic breast cancer cells [26]. Podophyllotoxin (PPT) is an organic pharmaceutical with anti-cancer properties in lung cancer and metastatic lung cancer. PPT could be captured in layered double hydroxides (LDH) nano-delivery systems and this approach demonstrates much better performance and lower toxicity in vivo in mice [27]. Harrison et al. [28] identified that a miRNA/circRNA regulatory axis promoted lung squamous carcinoma metastasis via CDR1-mediated regulation of golgi trafficking. They used a targeted lipid NP to deliver miR-671-5p to target the noncoding CDR1as/CDR1 axis and inhibited lymphatic metastatic spread in vivo in mice. Xu et al. [29] developed a systemic metastasis-targeted nanotherapeutic (H@CaPP) for co-delivering an anti-inflammatory agent, piceatannol, and an anti-thrombotic agent, low molecular weight heparin, to the primary tumor site to impede the multiple steps of tumor metastasis. This nanoformulation efficiently hindered EMT, inhibited the formation of “micro-thrombi”, and prevented the development of pre-metastatic niche. More so, in combination with surgical resection or chemotherapy, the above nanoformulation efficiently inhibited lung metastasis and prolonged overall survival of breast tumor-bearing mice. EGFR is a common oncogene, and the anti-EGFR monoclonal antibody cetuximab has been approved for treatment of metastatic colon cancer and head and neck squamous cell carcinoma. This antibody was investigated in conjugation with MRI-imageable multifunctional magnetic iron-oxide NPs (IONPs) administered via convection-enhanced delivery to glioblastoma (GBM). Cetuximab-IONP bound/internalized better to GBM cells and were increasingly uptaken by EGFR- as well as EGFRvIII-expressing GBM stem-like cells GSCs and neurospheres, thus demonstrating a significant increase in survival and a potential to halt tumor metastasis and recurrence [30]. A number of studies suggest that optimal size of therapeutic NPs is needed to extend the circulation time of therapeutics such that they distribute to and reach the target. In addition, many studies also provide examples for how nanotechnology paired with chemotherapy and other mechanisms increases the likelihood of a positive outcome to mitigate metastasis by targeting primary cancer. In this context, Liu et al.’s group developed tumor-specific CD44-targeted ideal-sized cationic bovine serum albumin (CBSA)-protected gold nanocluster and hyaluronic acid (HA) fabricated NPs (AuNC@CBSA@HA) for breast cancer targeting drug delivery and this formulation showed higher distribution in subcutaneous breast cancer. The group then decorated this formulation with nitric oxide (NO) donor to improve tumor blood supply and enhance tumor penetration and thereby tumor accumulation. Consequently, chemodrug paclitaxel (PTX) and indocyanine green (ICG)-loaded NPs could greatly inhibit primary tumor growth and prohibit lung metastasis [31,32,33,34,35]. This provides a theranostic strategy addressing the size, penetration, and toxicity concerns in drug delivery to tumor for improved anti-tumor/anti-metastasis effect. Similarly in another study, co-administration of tumor-homing peptide iRGD with multistage-responsive NPs dendri-graft-l-lysine conjugated with doxorubicin (DOX) and indocyanine (IDD) with NO donor-modified HA shell (HN) and laser irradiation could further improve tumor accumulation of NPs, thus resulting in better suppression of breast tumor growth and eventual metastasis [36]. Ceramide nanoliposomes also work as an adjunct to targeted chemotherapy in metastatic breast cancer and metastatic melanoma. The combination with Sorafenib synergistically inhibited proliferation and tumor growth in mice along with the demonstration of negligible systemic toxicity [37]. Furthermore, the afore-mentioned LDH can also serve for the delivery and increased effectiveness of standard chemotherapeutic agents such as Fluorouracil (5-FU) towards inhibiting cell growth of colon cancer cells [38].
NPs have also been used towards modulating primary TME as a promising strategy for cancer metastasis prevention and treatment. Several treatment strategies, such as radiotherapy, phototherapy, and vascular disrupting agents, could be used to elevate hypoxic microenvironment, since this hypoxia can contribute to tumor resistance and metastasis [39]. Temsirolimus is a mammalian target of the rapamycin (mTOR) inhibitor that can inhibit angiogenesis acting via hypoxia inducing factor-1 (HIF-1)α-mediated vascular endothelial growth factor (VEGF) expression [40]. To attenuate the hypoxia microenvironment, combretastatin A4 (a vascular disrupting agent)-loaded PLGA NPs (CA4-NPs) were combined with intraperitoneal injection of temsirolimus, and this co-treatment significantly reduced the expression of HIF-1α, reversed the immune suppression, and, as a result, reduced the lung metastasis rate [41].
Several studies also showed that institution of novel therapeutic strategies with nano-combination formulations for obliteration/suppression of metastatic tumor masses by focusing on cancer stem cells (CSCs) behavior could pave the way to improved treatment of several cancers. CSCs, also known as tumor stem cells (TSCs) or tumor initiating cells (TICs), are a small subset of immortal tumor cells that possesses the capacity to self-renew and cause the heterogeneous lineages of cancer cells that comprise the tumor [42]. CSCs can differentiate and evolve into tumor cells with a variety of phenotypes and are regulated by various key signaling pathways [43]. These pathways constitute a complex network of cellular interactions that facilitate both the initiation of the development of metastasis-propagator cells and pre-metastasis niche by the primary tumor and the formation of a nurturing organ microenvironment for migrating CSCs. CSCs are proposed to be the crucial driving force of tumor initiation, EMT, initiation of invasion and metastatic dissemination as well as of tumor recurrence [44]. Furthermore, CSCs can home far from tumor vessels, making it difficult for drug agents to target them and can also contribute to heterogeneity, chemoresistance and radioresistance of primary and metastatic tumors [45]. Such properties of CSCs emphasize their importance in metastatic progression, recurrence, and drug resistance mechanism. The surface markers expressed by CSCs provide a source for molecular targeted therapies for various cancers, for example, by using therapeutic antibodies specific for these markers. In this respect, nanomedicine has the potential to target CSCs for achieving effective cancer and metastasis treatment outcome. By recognizing specific properties of CSCs, various formulations were developed via designing effective nanodrugs, which specifically target CSCs in tumor tissues. For example, several next-generation nanotheranostics (NGNT) for both CSC-related therapy and diagnostics have been developed in the recent past. These multifunctional NPs have the ability to diagnose and deliver potent therapeutics to specific sites with the aid of targeting ligands or biomarkers in a “smartˮ way [46]. Zuo ZQ et al. [47] demonstrated enhanced tumor penetration of stem cell therapy drug-carrying NPs and CSCs clearance in vivo via synergistic TGF-β signaling pathway inhibition in mice bearing breast cancer xenografts, resulting in anti-tumor and anti-metastasis effects. Combination of thermo- and chemotherapy utilizing systemically administered silica-based multifunctional magnetic NPs that encapsulated a chemotherapeutic agent and coated with a specific antibody against the human lung CSCs have been used for effective in vivo suppression of lung tumor growth and metastasis in mice [48]. Controlled intratumoral and intracellular navigation using the polymeric micelle-based nanomedicine incorporating cisplatin (CDDP/m) was able to eradicate both undifferentiated cell and differentiated cancer cell populations within head and neck tumors [49]. This approach may provide an improvement in treating late-stage and metastatic cancer cases that suffer tumor relapse following cisplatin treatment. Further, in breast tumor mouse models, a convergent therapeutic strategy using a cell-differentiation-regulated nanomedicine to overcome the CSC-derived heterogeneity-imposed therapeutic barrier and to enhance the chemotherapeutic response, was examined. The study revealed that the NPs that are co-loaded with the differentiation-inducing agent, all-trans retinoic acid, and the chemotherapeutic drug, camptothecin (CPT) suppress tumor growth and prevent post-surgical tumor relapse and distant metastasis [50]. These findings emphasize the maximized synergistic anti-cancer efficacy of two drugs with distinct mechanisms of action to overcome intratumor heterogeneity and distant metastasis-associated therapeutic obstacles. Liu et al. [51] reported a combination of traditional chemotherapy, anti-CSC therapy, and immune checkpoint blockade cocktail therapy based on the spatio-temporally controlled nanodevice as a strategy for treating metastatic breast cancer in mice. The chemotherapeutic agent PTX, the anti-CSC agent thioridazine (THZ), and the programmed death (ligand) PD-1/PD-L1 inhibitor HY19991 (HY) are all incorporated into one drug delivery system, PM@THL, an enzyme/pH dual-sensitive NP with a micelle-liposome double-layer structure for improved drug delivery. Another nanomedicine based combinational chemotherapy strategy includes co-encapsulated HA-coated liposomes of cabazitaxel and CSC inhibitor silibinin to target CD44 receptors on CSCs [52]. Kaushik et al. [53] reported the connection between CSCs and EMT and how the latter is responsible for the generation of the former cells emphasizing the cell/process interconnection relevance in metastasis. They exposed GBM cells and mice-bearing xenografts to polyethylene glycol (PEG)-coated gold NPs (GNPs) and cold plasma. Simultaneous administration of low dose of these agents was able to delay tumor growth and prevent metastasis through PI3K/AKT inhibition pathway. This involved EMT reversal suggested by Slug and ZEB-1 down-regulation and the reduction in CD133 + cells following therapy, a marker for CSCs. Liu Y et al. [54] demonstrated in vivo reduction of pulmonary and hepatic micrometastases and provided evidence on intrinsic anti-tumoral effect of metallofullerenol NPs containing Gadolinium (Gd) on breast cancer cells by reversing EMT following TGF-β inhibition. The results were based on HIF-1α inhibition and decreased CSC population in that CSC reside in hypoxic tumor regions. Further, EpCAM is an epithelial cell adhesion molecule that is overexpressed in multiple carcinomas and CSCs. In this direction, Petersburg et al. [55] developed chemically self-assembled nanorings (CSANs) as prosthetic antigen receptors (PARs) for the nongenetic, rapid, stable and reversible modification of T cells surfaces. The use of PARs T cells to target solid tumors expressing the EpCAM and human CD3 receptor (αEpCAM/αCD3) was tested using an orthotopic breast cancer xenograft model as an alternative and complementary T-cell targeting approach. It was shown that these CSANs that are bispecific and polyvalent can guide and reversibly control cell–cell interactions, both in vitro and in vivo, and show promise for anti-cancer and anti-metastasis cell-directed immunotherapy.
Nano-immunotherapeutics for stimulating the host’s own immune defense and anti-tumor immunity response are another promising approach in treating cancer metastasis, as this may help settle the immunological tolerance and improve treatment efficacies. While some NPs possess inherent immuno-stimulating properties and can activate host immune systems against cancer and metastasis, other agents can also be loaded onto NPs and specifically delivered to the tumor site to activate local and distant immune response. Orally given Mica NPs steered macrophages and bone marrow-derived dendritic cells to modulate the local microenvironment to relieve immunosuppression and potentiate anti-tumor immunity against antigens expressed by the tumor in xenograft breast cancer metastatic mouse model. This change resulted in the suppression of tumor cell growth and subsequent metastases [56]. Selenium (Se) NPs-enriched lactobacillus administered orally, followed by subcutaneous injection, as preventative measure can increase host immune response (i.e., pro-inflammatory cytokines and NK cell cytotoxicity) and prolong the survival of breast cancer-bearing mice [57]. Rao et al. [58] addressed macrophage immunomodulation by combining a biomimetic synthetic strategy with cell-membrane-coating nanotechnology to inhibit melanoma and breast cancer distant metastasis in mice. Their findings demonstrate that genetically edited cell-membrane-coated magnetic NPs (gCM-MNs) can elicit potent macrophage immune responses for cancer immunotherapy by blocking the CD47-SIRPα signaling pathway. The MN core promotes the M2-to-M1 tumor-associated macrophage repolarization within the TME, synergistically triggering macrophage phagocytosis of cancer cells as well as anti-tumor T-cell immunity. In addition, the biomimetic gCM shell protects the MN core from immune clearance; and as a feedback mechanism, the MN core transports the gCM shell into the TME under magnetic navigation, improving the systemic circulation and tumor accumulation of gCM-MNs and reducing the off-target immune effects. Antigenic peptide CpG-ODN is a Toll-like receptor 9 (TLR9) agonist that is widely used in cancer immunotherapy to improve anti-tumor immune response. Liu et al. [59] developed a combination immunotherapy strategy that could promote stronger anti-tumor immune response. This strategy involved nanovaccine by self-assembly of CpG-ODN and cationic polymeric NPs loaded into hydrogel, as well as the nanomedicine encapsulated curcumin. While the nanovaccine augmented the anti-tumor T-cell immunity, the nanomedicine enhanced tumor immunogenicity induced cancer cell apoptosis. This combination strategy successfully delayed the growth of postoperative primary breast tumor and reduced the pulmonary metastasis in mice.
Combination of chemotherapy with immunotherapy and nanotechnology approaches has shown to stimulate strong anti-tumor immunity to treat tumor metastasis. To improve the immunotherapy efficiency of anti- PD-L1 antibody, Yang et al. [60] designed nanoassemblies combining glutathione-activatable drugs dimer-7-ethyl-10-hydroxycamptothecin (d-SN38) and dimer-lonidamine (d-LND)-coloaded bilirubin NPs (SL@BRNPs). With the facilitation of iRGD peptide, the SL@BRNPs could actively distribute in primary breast cancer and induce robust anti-tumor effect and immune response as well as prevent and/or treat lung metastasis. This is an example of a combination of activatable drug dimers and stimuli-responsive drug release for improved drug delivery. Similarly, Kuai et al. [61] reported high-density lipoprotein-mimicking nanodiscs to deliver DOX into tumor, which could boost the anti-tumor immune response and potentiate immune checkpoint blockade. DOX/indoximod (IND)-liposome design successfully inhibited liver metastasis of colorectal cancer in mice when combined with PD-L1 therapy. Lu et al. [62] designed an innovative nano-enabled approach by constructing a liposomal carrier by self-assembly of the phospholipid-conjugated prodrug, IND, which inhibits the indoleamine 2,3-dioxygenase (IDO-1) pathway, followed by the remote loading of DOX. This DOX/IND-liposome could suppress the breast cancer tumor growth and eradicate lung metastasis in vivo in mice with further combination of PD-L1 antibody therapy. Li et al. [63] designed a device formed by nanodiamonds with surface functionalization of polyglycerol loaded with DOX (Nano-DOX) to reprogram immunosuppressive TME of GBM and to induce anti-cancer and anti-metastasis immune response, where activation of autophagy, instead of apoptosis, was confirmed in Nano-DOX-treated GBM cells and in xenograft models. Several photothermal agents and NPs were developed to combine with checkpoint blockade immunotherapy to address tumor metastasis. Chen et al. [64] synthesized dual-loaded poly lactic-co-glycolic acid (PLGA) NPs (PLGA-ICG-R837) with ICG and imiquimod (R837) to treat in vivo breast tumor metastasis in mice by IV systemic administration in combination with anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4). This photothermal therapy with immune-adjuvant NPs and checkpoint blockade could stimulate stronger anti-tumor immune response and could suppress the growth of primary tumor, distant tumor, and tumor metastasis. Likewise, PDA-PEG-R848-CD NP, i.e., polydopamine loaded with resiquimod (R848) carbon dots, also showed effective liver and lung metastasis (of breast tumor) inhibition in mice when combined with PD-1 antibody therapy [65]. Taking a step even further by combining chemotherapy with photoimmunotherapy, as well as fluorescence and photoacoustic dual-modality imaging, a polypyrrole-loaded CPT-conjugated HA NP (P@CH) was developed for tumor targeting synergistic triple-combination therapy. When further combined with anti-PD-L1 antibody, the primary breast tumor in mice was completely depleted, and the lung metastasis was ablated [66]. Nanotechnology could also enhance the combination of photodynamic therapy with chemo-immunotherapy to produce anti-metastatic effect. Yu et al. [67] designed a hyaluronidase-responsive size-changeable biomimetic NPs (pPP-mCAuNCs@HA) with blood red cell membrane coating to deliver photosensitizer pheophorbide A, ROS-responsive chemotherapeutic PTX dimer prodrug (PXTK), and anti-PD-L1 peptide dPPA. The combination strategy enhanced penetration into deep breast tumor and deliver drug to tumor homogenously, produces high concentration of ROS following laser irradiation, and stimulates anti-tumor immune response. As a result, the lung metastasis was greatly inhibited, to the extent of no obvious lung metastasis. Similarly, Liu et al. [68, 69] identified that chlorin e6 (Ce6)-loaded macrophage-mimic shape changeable NPs or chimeric molecules that can form micelles could improve the accumulation of Ce6 and chemotherapeutics, thus stimulating strong anti-tumor immune response, and suppressing the lung metastasis of breast cancer in mice models. Further, delivering biomimetic nanoenzymes to tumor could also enhance synergistic photodynamic-immunotherapy and theranostics ability. The hypoxia-tropic nanoenzymes, such as MnO2-based NPs, could catalyze H2O2 to O2, improving oxygen level in hypoxic tumor and inhibiting metastasis [70]. Liu et al. [71] developed a photodynamic therapy method composed of co-delivery of IDO inhibitor with photosensitizer for boosting immune response and anti-metastasis ability. This consisted of a redox-activated porphyrin-based liposome nanovesicle strategy that has the potential for the synergistic immunotherapy that significantly suppressed primary tumor growth in vivo in mice, improved survival time, and induced effective breast cancer anti-tumor metastasis.
3 Nanotechnology-assisted targeting of invasion/intravasation
Targeting of the tumor cell-enhanced invasion into the surrounding tissues and nearby vessels is being investigated as a strategy to treat metastasis effectively. Nayak et al. [72] demonstrated that combination treatment with Nanoquinacrine (NQC) and ADAM-17 inhibitor (GW280264) decreased the invasion and proliferation rates in cervical CSCs. This combination approach induced Nectin-4 expression resulting in activation of base excision repair pathway and metastasis inhibition. The findings of this study unraveled a prominent role for Nectin-4 in 5-FU resistance of metastatic cervical cancer cells and that NQC sensitizes these cells, thus providing this nano-assisted approach as a useful strategy to overcome 5-FU chemoresistance. Further, the therapeutic and MRI-visible theranostic nanomedicine platform containing miR-125b-5p to target EMT and CSCs effectively demonstrated inhibition of tumor growth, invasion, and migration in vivo and in vitro in hepatocellular carcinoma (HCC) [73]. Luo et al. [74] synthesized a cathepsin B/pH dual-sensitive block copolymer to conjugate with DOX and to further load with nifuroxazide (NFX) to self-assemble as co-prodrug-loaded micelles (CLM). CLM reduced viability and inhibited migration and invasion of mouse breast cancer cells in vitro. Enhanced anti-tumor and anti-metastatic effects were found in breast cancer mice models following IV injection of CLM.
4 Nanotechnology-aided targeting of dissemination/mobility and migration
Disruption of migration of tumor cells and the binding of hetero-aggregates and the endothelium could prevent the next steps in metastatic cascade. Ding et al. [75] developed a versatile nano-platform by co-loading iron chelator Di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT) and cisplatin into intracellular drug-accumulating as-NPs for tumor-targeting therapy and metastasis inhibition. Dp44mT is a thiosemicarbazone chelator and has high Fe-binding affinity and membrane permeability, which could inhibit tumor cell mobility and migration. The Dp44mT and cisplatin-coloaded NPs exhibited enhanced intracellular drug accumulation and reduced side effects while suppressing the expression of HIF-1 and VEGF which participate in tumor invasion and migration. Consequently, in breast tumor-bearing mice, the above combination nanoplatform dramatically prevented orthotopic mammary tumor growth and inhibited lung metastasis. This study is an example for an approach where combination of different agents such as chemotherapy and iron chelators with a nano-carrier system works to tackle cases to efficiently inhibit tumor metastasis. Further, Liu et al. [76] reported that the 100-nm iCluster platform could distribute in breast tumor and reduce the size from 50 to 5 nm in response to the tumor acidity. This reduced size NPs could facilitate the improved perfusion of NPs and their transport from tumor to lymph nodes (LNs) and may inhibit lymph and lung metastasis, thus providing an effective delivery strategy of chemotherapeutics into the circulatory system LNs and circulating tumor cells (CTCs) through systemic administration.
CTCs are cancer cells that enter the lymphatic or blood vessels. CTC targeting, isolation, and analysis offer the possibility for early cancer detection, dynamic prognosis monitoring, as well as development of early metastasis combating strategies, all of which are reviewed elsewhere. For a detailed review on nanotechnology-assisted study of CTCs on microfluidic devices please refer to Cheng et al. [77]. Appropriately designed NPs can bind CTCs and reduce/arrest their migration and thus slow down metastatic spread. Izadi et al. [78] used carboxylated graphene oxide (CGO) conjugated with trimethyl chitosan (TMC) and HA NPs loaded with HIF-1α-siRNA and the CDK inhibitor, Dinaciclib, for silencing HIF-1α and blocking CDKs in CD44-expressing cancer cells. The NPs were shown to exhibit conceivable physicochemical properties, high cellular uptake, and low toxicity. Moreover, combination therapy of cancer cells using this formulation significantly suppressed the CDKs/HIF-1α and subsequently, decreased angiogenesis, proliferation, migration, and colony formation in tumor cells. These findings need to be validated further in in vivo conditions. Next, platelet membrane-coated biomimetic PLGA NPs (PMNPs) or nanoplatelets that co-encapsulated DOX, and the FDA-approved photothermal agent, ICG into the biomimetic nanoplatelets demonstrated improved adhesion to CTCs in lymphatics and reduced the lung metastasis of breast cancer in mice [79]. C6 ceramide nanoliposome was able to counteract migration and extravasation under shear conditions in metastatic melanoma and breast cancer cells, as well as in pancreatic cancer cells. The mechanisms underlying these effects were cytoskeletal remodeling, focal adhesion disassembly, and integrin αvβ3 affinity modulation [80, 81]. Zhu et al. [82] showed enhanced anti-migratory and anti-invasive effects of chemotherapy drug Etoposide delivered in LDH NPs (VP16-LDH) in comparison to free VP16 in lung cancer cells in vitro. Nanoparticles were conjugated with LyP-1, a peptide that can specifically bind to tumor and endothelial cells of tumor lymphatics and could annihilate tumor lymphatics. The conjugation resulted in intensified uptake in LNs and offers potential in the inhibition of lymphatic spread of tumors by targeting the nearby migrating cells [83].
5 Nanotechnology-supported targeting of the pre-metastatic niche and micrometastasis
Targeting early metastasis opens promising therapeutic avenues for metastasis prevention. As mentioned above, nanotherapeutic H@CaPP co-delivering piceatannol and heparin impedes EMT, the formation of “micro-thrombi”, and the development of pre-metastatic niche, thus inhibiting lung metastasis in breast tumor-bearing mice [29]. This could be a promising strategy; however, the use of anticoagulants as a specific metastasis prevention strategy should be considered with caution in human translation studies given the risk of bleeding complications. Metallofullerenol NPs containing Gd reverse EMT following TGF-β inhibition, modulate HIF-1α expression and CSC count, and thus reduce pulmonary and hepatic micrometastases in vivo in breast cancer-bearing mice [54]. Zhao et al. [84] conjugated S100A4 siRNA with CBSA and then coated them with exosome membrane (CBSA/siS100A4@Exosome) for targeted modulation of pre-metastasis niches in lung from postoperative breast cancer. CBSA/siS100A4@Exosome has a high affinity toward pre-metastasis niches and exhibited outstanding gene-silencing effects that significantly inhibited the growth of metastatic nodules in vivo. Furthermore, Peiris et al. [85] used a vascular targeting NP platform combined with radionuclide imaging theranostic strategy. This approach could target micrometastasis in vascular beds using GNPs in a mouse model of breast cancer metastasis.
6 Nanoparticle-enabled (macro)metastasis site-targeting drug delivery
Nanotechnology has been employed in many studies toward successful inhibition of tumor metastasis by directly targeting tumor cells or TME located in the metastasis site, to curb metastasis growth. Sun et al. [86] conjugated zoledronic acid (ZOL) a third-generation nitrogen-containing bisphosphonate, onto mesoporous silica NPs coated gold nanorods (Au@MSNs-ZOL) for targeting breast cancer bone metastasis as combination therapy. ZOL has strong affinity with bone and could drive NPs to bone metastasis, as well as induce tumor cell apoptosis, reduce VEGF level, and inhibit farnesyl pyrophosphate synthase which leads to the loss of osteoclasts. After intravenous injection to reach the metastatic site, Au@MSNs-ZOL was found more effective for bone metastasis photothermal therapy (achieved with laser light assistance) than unmodified NPs (Au@MSNs). Also, (Au@MSNs-ZOL + laser) could effectively suppress growth of bone metastasis and bone pain in a mouse model of intraosseous injection of MDA-MB231 breast cancer cells [86]. Likewise, alendronate, another commonly used bisphosphonate, was decorated onto polyamidoamine (PAMAM) dendrimer for bone metastasis targeting delivery of docetaxel (DTX@ALN-PAMAM). This combinational therapy was tested in vitro and in vivo in mice where it significantly reduced the size of bone metastasis of lung cancer as determined by micro-CT, as well as pain response [87]. Loading with small molecule transcription factor Gli2 inhibitor, the alendronate-modified NPs decreased tumor-associated bone lesion area and increased bone volume fraction in the tibiae of the mice. This drug modification balanced the bone-binding and led to the advantageous side effect of reduction of blood circulation time of NPs, i.e., causing the reduction of nonphagocytic phagocytosis of the formulation by macrophages. To overcome the challenge that bisphosphonates only improved affinity with bone tissue rather than tumor cells in bone metastasis, folic acid was anchored onto alendronate-modified PTX-loaded PLGA NPs for dual bone/tumor metastasis-targeted chemotherapy [88]. The presence of folic acid modification on NPs showed beneficial effect on breast cancer cell uptake in vitro. Similarly, in vivo, PTX-loaded NPs that had the folic acid modification displayed lower tumor growth rate and longer survival time than those that did not, indicating that the dual targeting delivery to improve tumor cellular uptake is beneficial for bone metastasis treatment. Further, LDH mentioned in the above sections can also be attached to a homing protein and can become multifunctional as it navigates the drug to the metastasis region by means of the homing protein. This approach can be a means to protect the drug from endonucleases and to deliver it to the nucleus [13, 89]. Brain treatments are hard-to-reach goal due to blood–brain barrier (BBB). In this direction, gene delivery NPs were engineered for targeted delivery to breast cancer brain metastasis by Zhou et al. with AMD3100, a small molecule antagonist of CXCR4 that is overexpressed in the brain metastasis tumor [90]. These modified NPs were made to artificially express secretory promelittin protein that is cleaved by tumor overexpressed MMP-2 to release cytolytic melittin and induce tumor cell apoptosis in mice. This study suggests a new direction to treat breast cancer brain metastasis through innovative targeted-delivery of promelittin-mediated gene therapy. Juthani et al. [91] propose that NP-drug conjugates (NDC) can be great candidates for targeting brain metastases with small molecule drugs, particularly in cases where the primary tumor is sensitive to the latter. Their findings lay foundation for the investigation of functionalized ultrasmall fluorescent core–shell silica NPs, Cornell prime dots (C’ dots) as potent drug delivery vehicles crossing the blood–brain barrier to treat both primary and metastatic CNS diseases while offering amplified drug accumulation at sites of disease and reduced off-target accumulations. An initial Phase 1 imaging study is now underway to investigate αv integrin-binding cRGD-C’ dot-accumulation in primary glioma or CNS metastasis patients [91].
In addition to functionalization of NPs with metastasis targeting ligand, biomimetic NPs could also represent a promising strategy for metastasis targeting drug delivery due to their specific interaction with tumor metastatic cells. As mentioned in the above section, Ye et al. [79] coated PLGA NPs with platelet membrane (PMNPs), referred to as nanoplatelets, and loaded with DOX and ICG for breast cancer lymphatic metastasis targeting therapy. When intravenously injected into mice, platelet membrane coating improved the adhesion to CTCs in the lymphatics and treatment with drug-loaded PMNPs reduced the lung metastasis. Overcoming the unintended toxicity to normal cells in multiple metastasis nodules is another promising and much needed strategy. To this end, Huo et al. reached down to organelle level and selectively eliminated liver metastatic cancer cells through metabolism-based energy depletion [92]. They conjugated nucleus-targeting W18O49 NPs (WONPs) to mitochondria-selective mesoporous silica NPs (MSNs) containing photosensitizer (Ce6) through an abnormally expressed Cathepsin B enzyme-cleavable peptide. The cleaved peptide linker allows WONPs and MSNs to respectively target nucleus and mitochondria, where the therapeutic powers could be unleashed, both photodynamically and photothermally in metastasis cells, while the abundant normal cells are spared.
Cancer-associated fibroblasts (CAFs) are a large component of the TME that contribute to tumor metastasis. CAFs constitute several types, such as the CAFs within primary tumors, and stromal fibroblasts at metastatic sites that can be termed metastasis-associated fibroblasts (MAFs). Both make great contributions to the establishment of metastatic lesions in that they can remodel the extracellular matrix (ECM) of metastatic tumors, modulate immune cells in the TME, promote angiogenesis, and augment malignant tumor phenotypes. MAFs can help establish pre-metastatic niches and mediate resistance to therapeutic strategies [93]. Activated MAFs increase tissue stiffness, which in turn triggers angiogenesis and promotes anti-angiogenic therapy resistance, indicating that MAFs are a promising target for metastatic cancer. Several NPs and nanoligands accomplished the transport of drugs to activated hepatic stellate cells (HSC)/myofibroblasts, the equivalent of CAF/(MAFs) in liver cancer. Surface modified nanocarriers with a cyclic peptide binding to the platelet-derived growth factor PDGFRβ, or with mannose-6-phosphate (M6P) binding to the insulin-like growth factor IGFRII, effectively directed drug delivery to activated HSC/CAF in vivo [94]. Similarly, unguided nanohydrogel particles and lipoplexes loaded with siRNA demonstrated a high in vivo uptake and functional siRNA delivery in activated HSC. Therefore CAF/HSC are an attractive target for the development of stroma-based therapies, both in stroma-rich cancers such as subtypes of HCC and pancreatic cancer PDAC, as well as in stroma-poor cancers, and can be addressed specifically by well-devised nanocarriers [94]. To know more on nanomedicine-based strategies to combat metastasis of stroma-rich pancreatic cancers, please refer to Li et al. [95]. Yin et al. [96] developed reduction-responsive polypeptide micelles based on methoxy PEG-block-poly(S-tert-butylmercapto-L-cysteine) copolymers to control the delivery of DOX in osteosarcoma therapy. Compared to free DOX, some of the micelle copolymers exhibited improved pharmacokinetics and tumor accumulation and decreased distribution in the heart. Moreover, the selective accumulation of some of the micelles in tumors induced stronger anti-tumor and anti-metastasis effects along with less systematic toxicity.
7 Nanotechnology-based imaging of metastases
Early identification of metastatic disease is fundamental prerequisite for planning its treatment. This can be quite challenging given the distance of metastases from the primary tumor site, initial undetectable size, low vascularization, and potentially limited contrast relative to its surrounding environment. Therefore, NP-based imaging agents are proving to be of added advantage in combination with standard imaging modalities to visualize metastatic lesions in various organs, as well as in therapeutics to deliver therapeutic agents. Added value of nanotechnology is ability to develop nanotheranostic strategies where agents are used for both detection and treatment.
NPs can be used to identify and visualize metastasis cells that are otherwise difficult to detect by conventional imaging technology only. Ongoing developments in the combination of MRI/PET/CT, which are the standard-of-care imaging methods for the detection of cancers, together with NP imaging have already made significant contributions. Nanoformulations such as multifunctional superparamagnetic iron oxide NP (SPIO, SPION) incorporating Gd for MRI contrast and utilizing antibodies or other targeting ligands have been developed to detect micro and macrometastases in a variety of organs following breast tumor metastasis [97]. Application of surfactant coatings, such as multifunctional nanotheranostic system based on poly(methacrylic acid)-polysorbate 80-grafted-starch, enable delivery of BBB-impermeable imaging and therapeutic agents and have extended this approach to predictive functional imaging, differential MRI diagnosis, and treatment of brain metastasis [98, 99]. SPIONs, specifically reporting on tumor vasculature and heterogeneity, were used in predicting the existence of brain metastases in melanomas [100, 101]. Absorption properties of iron oxide nanochain particles for fluorescence molecular tomography have been utilized in multimodal imaging where MRI is integrated with another imaging method towards vascular targeting to trace liver, lung, and brain metastases of breast cancer [102]. Furthermore, the newer (modified) generation of SPIO NPs was developed to exhibit multifunctional characteristics for theranostic applications [103]. Next, by employing nano-single photon emission computed tomography (SPECT), which is a high-sensitivity multi-pinhole SPECT, PET tracers were directed to metastatic lesions by conjugation to antibodies for surface markers associated with metastases, such as anti-carcinoembryonic antigen for tracking hepatic metastasis of colorectal cancer [104]. Further, as mentioned in the above sections, ultrasmall fluorescent core–shell silica NP, termed Cornell prime dots or C’ dots were assessed towards a dual-modality (PET-optical) platform for delivering native small molecule drugs to CNS metastasis [91]. Similar spectrally distinct near-infrared (NIR) fluorescent or iodine-radiolabeled C′ dots were used to monitor and multimodally image nodal metastases of melanoma [105]. Diocou et al. [106] performed radionuclide imaging with [18F]tetrafluoroborate ([18F]BF4−) (PET/CT) and found that the PET radiotracer is useful for sensitive and specific metastasis detection with excellent contrast in an orthotopic xenograft breast cancer model. Human sodium iodide symporter (NIS) was expressed as a reporter, and [18F]BF4− was found superior compared to the conventional tracer [123I]iodide (sequential SPECT/CT) due to faster tumor uptake as well as quicker and complete clearance from circulation. This technique is suggested to be useful whenever preclinical in vivo cell tracking is of interest. PET/MRI agents with inherently multimodal, all-organic NPs have also been developed as “porphysomes” and directed to prostate cancer bone metastases in vivo in mice [107]. MR/X-ray contrast-bearing theranostic NPs (TNPs) comprising Gd-shell-coated Au nanorods were used for site-directed photothermal therapy of colorectal cancer liver metastasis towards interventional radiology. The uptake of TNPs with hepatic delivery was found to be double compared to that of systemic administration and provided better thermal damage of metastases [108]. Albumin-based theranostic nano-probe HSA-Gd-IR825 was developed as a molecularly targeted approach to image a malignant lesion, and this was combined with photothermal ablation of metastases traversing through the lymph post-surgery [109]. Further, the anti-HER2 antibody can also be conjugated onto W18O49 NPs for theranostic purpose, i.e., for targeted X-ray CT guided imaging and photothermal ablation of breast cancer lymphatic metastasis [110].
Moving to other imaging modalities and their combination with NPs, in vivo optical imaging requires brightly emitting, tissue-specific materials that optically transmit through living tissue and can be imaged with portable systems that display data in real-time. Nevertheless, insights into tumor growth, macrometastasis, and tumor angiogenesis as well as functional readouts of subcellular biological processes such as protein–protein interactions are provided by non-invasive molecular imaging using fluorescent probes and multi-photon microscopy [111, 112]. Although such strategies have helped the advancement of the study of cancer dynamics in situ, drawbacks relating to interference with tissue absorption and auto-fluorescence leading to low sensitivity of detection of exogenously labeled cells continue to restrict adequate resolution in vivo. In this context, Naczynski et al. [113] have used human serum albumin encapsulated inorganic-protein nanocomposites rare-earth (ReANC) NPs to detect emerging and disseminated tumors in melanoma mouse models. These highly luminescent Re nanomaterials offer superior detection sensitivity over other short wave IR region (SWIR) emitters while offering the capability of improved anatomical resolution of multispectral in vivo imaging. This offers opportunity for improved in vivo optical imaging for disease screening and image-guided surgical interventions. Kantamneni et al. [114] demonstrated the unique capabilities of erbium-doped ReANCs for surveillance of multi-organ metastases in mice of basal human breast cancer using a cocktail of niche-targeted probes with excellent safety and clearance profiles. Lack of sufficient contrast between the diseased lesion and healthy tissue is one stumbling block on the usage of optical imaging fluorophores. To overcome this, methods such as albumin nanoshells targeting ligands have been utilized for improved targeting of functionalized Re-albumin nanocomposites. The albumin nanoshells can be either adsorbed directly via the drug binding pockets inherent to albumin or by chemical bioconjugation, providing enhanced contrast, enhanced cancer cytotoxicity, and minimal collateral damage to healthy primary cells and have been evaluated in melanoma tumor spheroid models [115, 116]. Bennett et al. [117] synthesized a series of ICG-conjugated ultra-pH sensitive polymeric micellar NPs to detect and resect (micro)metastasis in LNs with image-guidance in a mouse breast cancer model. In an effort to synthesizing the future Nanobots, Zheng et al. [118] developed iridium-based hypoxia-activated optical oxygen nanosensor. Upon testing in cell and animal models, micelle nanosensor gave strong signals in tissue with metastases by both whole-body imaging and organ imaging and exhibited good biocompatibility. This nanosensor can be selectively activated in the hypoxic microenvironment and be effectively delivered to the metastasis site through bloodstream or lymphatics, thus offering a powerful tool for the diagnosis of cancer metastasis. Molabaasi et al. [119] developed a biocompatible platform by affixing HA onto luminescent platinum nanoclusters (Pt NCs) in human hemoglobin (Hb) (Hb/Pt NCs). This bioplatform could serve as an efficient theranostic strategy for targeting of CD44-overexpressing cancer cells and CSCs. Further, radionuclide imaging has also been used for imaging of radio-labeled dual-ligand NPs in metastatic breast cancer. For example, the gamma scintigraphy was carried out using Technetium-99 m (99mTc) as a radionuclide label for NPs, and scintigraphy imaging showed that vascular targeting resulted in early-stage metastasis “hot spots” in the lungs of mice with breast cancer metastasis [120]. Similarly, through labelling with 99mTC and a αvβ3 targeting ligand, the GNPs could target micrometastasis in vascular beds in the mouse model of breast cancer metastasis at a low dose using radionuclide imaging [85]. One other promising approach to improve the effectiveness of radiotherapy is the use of radiosensitizing NPs with both imaging and therapeutic properties on the same nano-object. Verry et al. [121] recently published MRI results of a phase 1 clinical trial where they performed radiosensitization with a single IV administration of ultrasmall Gd-based AGuIX NPs followed by radiotherapy in patients with brain metastases from melanoma, lung, colon, and breast cancer. The NPs accumulated and enhanced image contrast in all types of brain metastases examined, and the MRI enhancements observed were equivalent in comparison with a commercial clinical MRI contrast agent. This trial provides one good example of a successful translation of this theranostic NP agent from the preclinical to the clinical level, as it is underway for a phase 2 clinical trial.
8 Nanomedicine translation—past and future
The research activity in nanomedicine over last two decades has been unprecedented. The rate of publications in this field went from almost zero in early 2000 to close to 30,000 publications per year most recently, as per PubMed search for publications containing keyword “nanoparticle.ˮ Many argue [122, 123] that prolific academic research did not translate into significant outcomes in clinical utility. There has been, however, slow trickle of Food and Drug Administration (FDA) approvals of nano-based drugs in the USA. Early market introduction of Doxil (1995) and Abraxane (2005) for cancer has been followed by Marqibo, Onivyde, and Vyxeos—all liposomal formulations of cytotoxic drugs [124,125,126,127]. Nanotherm (particles for hyperthermia treatment of glioblastoma) and Hensify (hafnium oxide nanoparticles for radiotherapy of soft tissue sarcoma) were approved in Europe [128, 129]. For a latest complete list of cancer nanomedicines with granted regulatory approval, and that are undergoing clinical trials, please see Table 1 and Supplementary Material in this review, and also refer to de Lazaro and Mooney, Kemp and Kwon, and Ventola [130,131,132].
It is true that these nanomedicines, which reduced life-threatening toxicities of the treatment, have demonstrated only modest improvement in the overall survival of patients [130,131,132]. Did this occur due to inherently limited potential of nanotechnology-based approach to improve the survival or due to the selection of disease targets, Active Pharmaceutical Ingredients (APIs), and translational strategies? First, several NP deliveries have been developed initially in engineering laboratories and early translation efforts attempted to use them as ‘platform technologies’ for treatment of different cancers and/or diseases with a belief that ‘one size can fit all’. This naïve belief is gradually abandoned with designs driven by a particular clinical application. Second, new medical technologies often suffer from risk-aversion at its early stage of development. As such, several maturing nanomedicines, which are being commercialized predominantly by small start-up companies, have carefully used established NPs (i.e., liposomes) and proven APIs (i.e., paclitaxel, doxorubicin) to improve odds of successful translation. Nanotherm and Hensify therapies, mentioned above, are first entries into the market which use inherent material property of NP for the therapeutic outcome rather than its ability to serve as a delivery vehicle for drug molecule. It was argued [133] that leveraging further materials properties of different NP constructs will contribute to increased innovation level of nanomedicines and potentially will increase their therapeutic efficacy. Similar is true with the selection of APIs – so far most of the NPs were used to deliver small molecule drugs used in chemotherapy. Recent (2018) approval of ONPATTRO™ (patisiran, Alnylam Pharmaceuticals), an RNAi therapeutic agent for the treatment of the polyneuropathy in amyloidosis, although not cancer application, showed the opportunity for expanding the repertoire of therapeutic molecules which can be delivered successfully using NPs [134].
Further improvements, which potentially can improve translational success and utility of nanomedicines include (1) the development of companion diagnostics jointly with incorporating imaging modalities into clinical trials to facilitate patient stratification and selection of those who could benefit from nanomedicine treatment most; (2) identification of ‘niche’ medical applications which can be addressed by nanotechnology and do not have viable contemporary solutions; (3) combining nano-therapies with other treatment modalities (not necessarily nano-based) for the efficacy enhancement; (4) improvements in nanomedicine manufacturing processes and quality control; and (5) establishing regulatory guidelines that specifically apply to nanomedical products [130,131,132, 135,136,137].
In summary, translational efforts in cancer nanotechnology have not delivered on the initial, most likely overrated promise. However, they have been steadily growing and deliver New Drug Application (NDA) and approvals every few years. The inspection of iSearch—NIH grant database as mentioned earlier—demonstrated that in the past 5 years, 14% of all cancer nanotechnology grants are focused on clinical efforts. An inspection of clinicaltrials.gov using “cancerˮ and “nanoparticleˮ as key words reveals 327 registered trials with 128 of them currently underway (see Supplementary Material). Once, Early Phase 1 and Phase I trials are excluded from this ‘currently underway’ search, we are still left with 70 trials of Phase II and 14 trials of Phase III. This is not unreasonable for a relatively small field of research. To put this into further context, we repeat what we indicated in the introduction. Clinically worthy anti-cancer approaches are very difficult to develop as indicated by analysis of NIH cancer-related grants. The limited entry of these approaches into the clinic is true across all technologies and treatment modalities and is not characteristic to nanotechnology, only.
To close, we should not forget about recent and rather spectacular success of NP delivery. mRNA-based Pfizer/BioNTech and Moderna COVID-19 vaccines have been successful due to employing lipid NPs for their delivery [138]. In the USA alone, these two vaccines administered ~ 500 M doses over the period of a year! The experience with their delivery, undoubtfully, will open door to mRNA-based cancer therapies in the future.
9 Conclusions
This contribution provides an overview of the research findings that shed light on nanotechniques/nanotechnology-assisted approaches that are currently being used/explored to counteract cancer metastasis. We highlight, in particular, the added value that nanotechnology-aided approaches are able to bring, either alone or in combination with the existing conventional techniques, to improve combating of the challenging and complex metastatic disease. The unique approach in the discussion presented here is to tie specific nanotechnology strategies to complex biology of different stages of metastatic cascade. As outlined in the various sections above, and in Fig. 1 and Table 2, several avenues of combating cancer metastasis, such as its diagnosis, prevention and treatment in the several metastatic cascade steps have significantly benefited from nanotechnology. In Fig. 1, we summarize different sections of this contribution, by assigning different attributes of nanotechnology approaches to different elements of metastatic cascade and capturing relevant references. Table 2 provides more detailed insight into specific techniques discussed in referenced publications and demonstrating their broad potential in combating metastasis.
Overall, nanotechnology-based approaches offer hopeful combination tools towards the targeting and disrupting of multiple steps of metastatic cascade thus presenting an opportunity for new therapies with potential for interruption or prevention of the next steps in metastatic cascade. Although the side effects and long-term effects of nanomaterials in the biological system are still under gauge, all the above reviewed data suggest nanotechnology as a promising tool for modulation, counteracting and efficiently treating the metastatic process with a potential to radically change the treatment pattern. Nanotechnology has made a significant impact on the selective recognition of the cancerous and other target cells; successful combating of tumor metastasis by targeting the various sites towards multiple measures such as inducing apoptosis of tumor cells; targeting the CSCs and CTCs, pre-metastatic niche and micrometastasis; hindering EMT; modulating the TME; stimulating immune responses; addressing tumor heterogeneity; enabling targeted and controlled drug delivery; and increasing the timely drug localization and cellular uptake. Nanotechnology also showed its flexibility in facilitating synergistic effects with multimodal conventional therapies overcoming some of the pitfalls associated with the latter, and also reaching the hard-to-reach goals by innovative targeted-delivery methods across biological barriers and the ability to develop nanotheranostic strategies. A more detailed insight into targeting EMT and angiogenesis could offer better opportunities for more improved outcome in halting the metastatic process. By using various types of NPs for the improved delivery of the accurate amount of drug to the target, without disturbing the physiology of the normal cells, the application of nanomedicine and nano-drug delivery systems are certainly the trend that will remain to be the future arena of research and development and promising translational efforts.
References
Caswell, D. R., & Swanton, C. (2017). The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Medicine, 15(1), 133.
Lüönd, F., Tiede, S., & Christofori, G. (2021). Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. British Journal of Cancer, 125(2), 164–175.
Saxena, M., Christofori G. (2013). Rebuilding cancer metastasis in the mouse. Molecular Oncology, 7.
van Zijl, F., Krupitza, G., & Mikulits, W. (2011). Initial steps of metastasis: Cell invasion and endothelial transmigration. Mutation Research, 728(1–2), 23–34.
Seyfried, T. N., & Huysentruyt, L. C. (2013). On the origin of cancer metastasis. Critical Reviews in Oncogenesis, 18(1–2), 43–73.
Anderson, R. L., et al. (2019). A framework for the development of effective anti-metastatic agents. Nature Reviews Clinical Oncology, 16(3), 185–204.
Fontebasso, Y., & Dubinett, S. M. (2015). Drug Development for Metastasis Prevention. Critical Reviews in Oncogenesis, 20(5–6), 449–473.
Jayne, D. G. (2003). The molecular biology of peritoneal carcinomatosis from gastrointestinal cancer. Ann Acad Med Singap, 32(2), 219–225.
Langley, R. R., & Fidler, I. J. (2011). The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. International Journal of Cancer, 128(11), 2527–2535.
Mollica, H., et al. (2019). Two-Channel Compartmentalized Microfluidic Chip for Real-Time Monitoring of the Metastatic Cascade. ACS Biomaterials Science & Engineering, 5(9), 4834–4843.
Redig, A. J., & McAllister, S. S. (2013). Breast cancer as a systemic disease: A view of metastasis. Journal of Internal Medicine, 274(2), 113–126.
Neben, K., et al. (2008). Metastases in the Absence of a Primary Tumor: Advances in the Diagnosis and Treatment of CUP Syndrome. Deutsches Ärzteblatt International, 105(43), 733–740.
Visentin, S., et al. (2020). Targeting Tumour Metastasis: The Emerging Role of Nanotechnology. Current Medicinal Chemistry, 27(8), 1367–1381.
Park, G. T., & Choi, K. C. (2016). Advanced new strategies for metastatic cancer treatment by therapeutic stem cells and oncolytic virotherapy. Oncotarget, 7(36), 58684–58695.
Wu, Q., et al. (2014). Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Letters, 347(2), 159–166.
Perez-Herrero, E., & Fernandez-Medarde, A. (2015). Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. European Journal of Pharmaceutics and Biopharmaceutics, 93, 52–79.
Ali, E. S., et al. (2021). Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives. Seminars in Cancer Biology, 69, 52–68.
Peer, D., et al. (2007). Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology, 2(12), 751–760.
Mocan, T., et al. (2017). Carbon nanotubes as anti-bacterial agents. Cellular and Molecular Life Sciences, 74(19), 3467–3479.
Mocan, T., et al. (2015). In Vitro Administration of Gold Nanoparticles Functionalized with MUC-1 Protein Fragment Generates Anticancer Vaccine Response via Macrophage Activation and Polarization Mechanism. Journal of Cancer, 6(6), 583–592.
Li, Y., et al. (2018). Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer-Challenges and Opportunities. Nanomaterials (Basel), 8(6).
van der Meel, R., Lammers, T., & Hennink, W. E. (2017). Cancer nanomedicines: Oversold or underappreciated? Expert Opinion on Drug Delivery, 14(1), 1–5.
van der Meel, R., et al. (2019). Smart cancer nanomedicine. Nature Nanotechnology, 14(11), 1007–1017.
Lungu, II, et al. (2019). Nanobiomaterials Used in Cancer Therapy: An Up-To-Date Overview. Molecules, 24(19).
BioparmaPEG. Current Nanomedicines for the Treatment of Cancer. 2021; Available from: https://www.biochempeg.com/article/188.html.
Stover, T., & Kester, M. (2003). Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. Journal of Pharmacology and Experimental Therapeutics, 307(2), 468–475.
Choi, S. J., & Choy, J. H. (2011). Layered double hydroxide nanoparticles as target-specific delivery carriers: Uptake mechanism and toxicity. Nanomedicine (London, England), 6(5), 803–814.
Harrison, E. B., et al. (2020). A Circle RNA Regulatory Axis Promotes Lung Squamous Metastasis via CDR1-Mediated Regulation of Golgi Trafficking. Cancer Research, 80(22), 4972–4985.
Xu, M., et al. (2021). Systemic metastasis-targeted nanotherapeutic reinforces tumor surgical resection and chemotherapy. Nature Communications, 12(1), 3187.
Kaluzova, M., et al. (2015). Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget, 6(11), 8788–8806.
Liu, R., et al. (2019). Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm Sin B, 9(2), 410–420.
Luo, Z., Dai, Y., & Gao, H. (2019). Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm Sin B, 9(6), 1099–1112.
Liu, R., et al. (2018). Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. Journal of Controlled Release, 278, 127–139.
Qin, L., & Gao, H. (2019). The application of nitric oxide delivery in nanoparticle-based tumor targeting drug delivery and treatment. Asian Journal of Pharmaceutical Sciences, 14(4), 380–390.
Zhang, W., et al. (2020). The progress and perspective of nanoparticle-enabled tumor metastasis treatment. Acta Pharmaceutica Sinica B, 10(11), 2037–2053.
Hu, C., et al. (2018). Coadministration of iRGD with Multistage Responsive Nanoparticles Enhanced Tumor Targeting and Penetration Abilities for Breast Cancer Therapy. ACS Applied Materials & Interfaces, 10(26), 22571–22579.
Tran, M. A., et al. (2008). Combining nanoliposomal ceramide with sorafenib synergistically inhibits melanoma and breast cancer cell survival to decrease tumor development. Clinical Cancer Research, 14(11), 3571–3581.
Chen, J., et al. (2014). Effective inhibition of colon cancer cell growth with MgAl-layered double hydroxide (LDH) loaded 5-FU and PI3K/mTOR dual inhibitor BEZ-235 through apoptotic pathways. International Journal of Nanomedicine, 9, 3403–3411.
Guan, X. (2015). Cancer metastases: Challenges and opportunities. Acta Pharm Sin B, 5(5), 402–418.
Del Bufalo, D., et al. (2006). Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Research, 66(11), 5549–5554.
Yu, H., et al. (2020). Tumor regression and potentiation of polymeric vascular disrupting therapy through reprogramming of a hypoxia microenvironment with temsirolimus. Biomater Sci, 8(1), 325–332.
Clarke, M. F., et al. (2006). Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Research, 66(19), 9339–9344.
Dreesen, O., & Brivanlou, A. H. (2007). Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev, 3(1), 7–17.
Li, F., et al. (2007). Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Research, 17(1), 3–14.
Baumann, M., Krause, M., & Hill, R. (2008). Exploring the role of cancer stem cells in radioresistance. Nature Reviews Cancer, 8(7), 545–554.
Reda, A., Hosseiny, S., & El-Sherbiny, I. M. (2019). Next-generation nanotheranostics targeting cancer stem cells. Nanomedicine, 14(18), 2487–2514.
Zuo, Z. Q., et al. (2016). Promoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-beta signaling pathway inhibition. Biomaterials, 82, 48–59.
Liu, D., et al. (2020). Targeted destruction of cancer stem cells using multifunctional magnetic nanoparticles that enable combined hyperthermia and chemotherapy. Theranostics, 10(3), 1181–1196.
Wang, M., et al. (2016). Eradication of CD44-variant positive population in head and neck tumors through controlled intracellular navigation of cisplatin-loaded nanomedicines. Journal of Controlled Release, 230, 26–33.
Shen, S., et al. (2021). A nanotherapeutic strategy to overcome chemotherapeutic resistance of cancer stem-like cells. Nature Nanotechnology, 16(1), 104–113.
Lang, T., et al. (2019). Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv Mater, 31(5), e1806202.
Mahira, S., et al. (2019). Cabazitaxel and silibinin co-encapsulated cationic liposomes for CD44 targeted delivery: A new insight into nanomedicine based combinational chemotherapy for prostate cancer. Biomedicine & Pharmacotherapy, 110, 803–817.
Kaushik, N. K., et al. (2016). Low doses of PEG-coated gold nanoparticles sensitize solid tumors to cold plasma by blocking the PI3K/AKT-driven signaling axis to suppress cellular transformation by inhibiting growth and EMT. Biomaterials, 87, 118–130.
Liu, Y., et al. (2015). Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nature Communications, 6(1), 5988.
Petersburg, J. R., et al. (2018). Eradication of Established Tumors by Chemically Self-Assembled Nanoring Labeled T Cells. ACS Nano, 12(7), 6563–6576.
Kang, T.-W., et al. (2015). Mica Nanoparticle, STB-HO Eliminates the Human Breast Carcinoma Cells by Regulating the Interaction of Tumor with its Immune Microenvironment. Scientific Reports, 5(1), 17515.
Yazdi, M. H., et al. (2012). The preventive oral supplementation of a selenium nanoparticle-enriched probiotic increases the immune response and lifespan of 4T1 breast cancer bearing mice. Arzneimittel-Forschung, 62(11), 525–531.
Rao, L., et al. (2020). Activating Macrophage-Mediated Cancer Immunotherapy by Genetically Edited Nanoparticles. Advanced Materials, 32(47), 2004853.
Liu, X., et al., Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials, 2020. 230: p. 119649.
Yang, X., et al. (2019). Tumor Microenvironment-Responsive Dual Drug Dimer-Loaded PEGylated Bilirubin Nanoparticles for Improved Drug Delivery and Enhanced Immune-Chemotherapy of Breast Cancer. Advanced Functional Materials, 29(32), 1901896.
Kuai, R., et al., Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Science Advances, 2018. 4(4): p. eaao1736.
Lu, J., et al. (2018). Breast Cancer Chemo-immunotherapy through Liposomal Delivery of an Immunogenic Cell Death Stimulus Plus Interference in the IDO-1 Pathway. ACS Nano, 12(11), 11041–11061.
Li, T. F., et al. (2019). Doxorubicin-polyglycerol-nanodiamond composites stimulate glioblastoma cell immunogenicity through activation of autophagy. Acta Biomaterialia, 86, 381–394.
Chen, Q., et al. (2016). Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nature Communications, 7(1), 13193.
Lu, Q., et al. (2019). Photothermally activatable PDA immune nanomedicine combined with PD-L1 checkpoint blockade for antimetastatic cancer photoimmunotherapy. Journal of Materials Chemistry B, 7(15), 2499–2511.
Sun, W., et al., Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials, 2019. 217: p. 119264.
Yu, W., et al., Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials, 2019. 217: p. 119309.
Liu, R., et al. (2020). Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. Journal of Controlled Release, 321, 589–601.
Liu, R., et al. (2019). Linear Chimeric Triblock Molecules Self-Assembled Micelles with Controllably Transformable Property to Enhance Tumor Retention for Chemo-Photodynamic Therapy of Breast Cancer. Advanced Functional Materials, 29(23), 1808462.
Gao, F., et al., Hypoxia-tropic nanozymes as oxygen generators for tumor-favoring theranostics. Biomaterials, 2020. 230: p. 119635.
Liu, D., et al. (2019). Redox-Activated Porphyrin-Based Liposome Remote-Loaded with Indoleamine 2,3-Dioxygenase (IDO) Inhibitor for Synergistic Photoimmunotherapy through Induction of Immunogenic Cell Death and Blockage of IDO Pathway. Nano Letters, 19(10), 6964–6976.
Nayak, A., et al. (2019). Nanoquinacrine sensitizes 5-FU-resistant cervical cancer stem-like cells by down-regulating Nectin-4 via ADAM-17 mediated NOTCH deregulation. Cellular Oncology (Dordrecht), 42(2), 157–171.
Guo, R., et al. (2019). Development of a Non-Coding-RNA-based EMT/CSC Inhibitory Nanomedicine for In Vivo Treatment and Monitoring of HCC. Advanced Science, 6(9), 1801885.
Luo, L., et al. (2020). Stimuli-responsive polymeric prodrug-based nanomedicine delivering nifuroxazide and doxorubicin against primary breast cancer and pulmonary metastasis. Journal of Controlled Release, 318, 124–135.
Ding, F., et al. (2020). Enhancing the chemotherapeutic efficacy of platinum prodrug nanoparticles and inhibiting cancer metastasis by targeting iron homeostasis. Nanoscale Horiz, 5(6), 999–1015.
Liu, J., et al. (2019). Enhanced Primary Tumor Penetration Facilitates Nanoparticle Draining into Lymph Nodes after Systemic Injection for Tumor Metastasis Inhibition. ACS Nano, 13(8), 8648–8658.
Cheng, J., et al., Nanotechnology-Assisted Isolation and Analysis of Circulating Tumor Cells on Microfluidic Devices. Micromachines (Basel), 2020. 11(8).
Izadi, S., et al. (2020). Codelivery of HIF-1alpha siRNA and Dinaciclib by Carboxylated Graphene Oxide-Trimethyl Chitosan-Hyaluronate Nanoparticles Significantly Suppresses Cancer Cell Progression. Pharmaceutical Research, 37(10), 196.
Ye, H., et al. (2019). Bioinspired nanoplatelets for chemo-photothermal therapy of breast cancer metastasis inhibition. Biomaterials, 206, 1–12.
Morad, S. A., et al. (2016). Short-chain ceramides depress integrin cell surface expression and function in colorectal cancer cells. Cancer Letters, 376(2), 199–204.
Krishnamurthy, K., et al. (2008). Deoxycholate promotes survival of breast cancer cells by reducing the level of pro-apoptotic ceramide. Breast Cancer Research, 10(6), R106.
Zhu, Y., et al. (2015). Enhanced Anti-Metastatic Activity of Etoposide Using Layered Double Hydroxide Nano Particles. Journal of Biomedical Nanotechnology, 11(12), 2158–2168.
Luo, G., et al. (2010). LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors. International Journal of Pharmaceutics, 385(1–2), 150–156.
Zhao, L., et al. (2020). Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. Journal of Controlled Release, 318, 1–15.
Peiris, P. M., et al. (2015). Vascular Targeting of a Gold Nanoparticle to Breast Cancer Metastasis. Journal of Pharmaceutical Sciences, 104(8), 2600–2610.
Sun, W., et al. (2019). Bone-Targeted Nanoplatform Combining Zoledronate and Photothermal Therapy To Treat Breast Cancer Bone Metastasis. ACS Nano, 13(7), 7556–7567.
Bai, S. B., et al. (2020). Bone-targeted PAMAM nanoparticle to treat bone metastases of lung cancer. Nanomedicine (London, England), 15(9), 833–849.
Chen, S.-H., et al. (2020). Alendronate/folic acid-decorated polymeric nanoparticles for hierarchically targetable chemotherapy against bone metastatic breast cancer. Journal of Materials Chemistry B, 8(17), 3789–3800.
Kraljevic, S. and K. Pavelic, Navigare necessere est. Improved navigation would help to solve two crucial problems in modern drug therapy: toxicity and precise delivery. EMBO Rep, 2005. 6(8): p. 695–700.
Zhou, Y., et al. (2020). Targeted Delivery of Secretory Promelittin via Novel Poly(lactone-co-β-amino ester) Nanoparticles for Treatment of Breast Cancer Brain Metastases. Advanced Science, 7(5), 1901866.
Juthani, R., et al. (2020). Ultrasmall Core-Shell Silica Nanoparticles for Precision Drug Delivery in a High-Grade Malignant Brain Tumor Model. Clinical Cancer Research, 26(1), 147–158.
Huo, D., et al. (2019). Eradication of unresectable liver metastasis through induction of tumour specific energy depletion. Nature Communications, 10(1), 3051.
Wang, Z., et al. (2021). Metastasis-associated fibroblasts: An emerging target for metastatic cancer. Biomark Res, 9(1), 47.
Kaps, L. and D. Schuppan, Targeting Cancer Associated Fibroblasts in Liver Fibrosis and Liver Cancer Using Nanocarriers. Cells, 2020. 9(9).
Li, Y. J., et al. (2020). Emerging nanomedicine-based strategies for preventing metastasis of pancreatic cancer. Journal of Controlled Release, 320, 105–111.
Yin, F., et al., Reduction-responsive polypeptide nanomedicines significantly inhibit progression of orthotopic osteosarcoma. Nanomedicine, 2020. 23: p. 102085.
Kievit, F. M., et al. (2012). Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS Nano, 6(3), 2591–2601.
Li, J., et al. (2014). A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS Nano, 8(10), 9925–9940.
Patil, R., et al. (2015). MRI virtual biopsy and treatment of brain metastatic tumors with targeted nanobioconjugates: Nanoclinic in the brain. ACS Nano, 9(5), 5594–5608.
Sundstrom, T., et al. (2013). Automated tracking of nanoparticle-labeled melanoma cells improves the predictive power of a brain metastasis model. Cancer Research, 73(8), 2445–2456.
Weinstein, J. S., et al. (2010). Superparamagnetic iron oxide nanoparticles: Diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. Journal of Cerebral Blood Flow and Metabolism, 30(1), 15–35.
Peiris, P. M., et al. (2012). Imaging metastasis using an integrin-targeting chain-shaped nanoparticle. ACS Nano, 6(10), 8783–8795.
Li, K., Nejadnik, H., & Daldrup-Link, H. E. (2017). Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discovery Today, 22(9), 1421–1429.
Rajkumar, V., et al. (2015). Texture analysis of (125)I-A5B7 anti-CEA antibody SPECT differentiates metastatic colorectal cancer model phenotypes and anti-vascular therapy response. British Journal of Cancer, 112(12), 1882–1887.
Chen, F., et al., Molecular phenotyping and image-guided surgical treatment of melanoma using spectrally distinct ultrasmall core-shell silica nanoparticles. Sci Adv, 2019. 5(12): p. eaax5208.
Diocou, S., et al. (2017). [(18)F]tetrafluoroborate-PET/CT enables sensitive tumor and metastasis in vivo imaging in a sodium iodide symporter-expressing tumor model. Science and Reports, 7(1), 946.
Liu, T. W., et al. (2013). Inherently multimodal nanoparticle-driven tracking and real-time delineation of orthotopic prostate tumors and micrometastases. ACS Nano, 7(5), 4221–4232.
Parchur, A. K., et al. (2018). Vascular Interventional Radiology-Guided Photothermal Therapy of Colorectal Cancer Liver Metastasis with Theranostic Gold Nanorods. ACS Nano, 12(7), 6597–6611.
Chen, Q., et al. (2014). An albumin-based theranostic nano-agent for dual-modal imaging guided photothermal therapy to inhibit lymphatic metastasis of cancer post surgery. Biomaterials, 35(34), 9355–9362.
Huo, D., et al. (2014). X-ray CT guided fault-free photothermal ablation of metastatic lymph nodes with ultrafine HER-2 targeting W18O49 nanoparticles. Biomaterials, 35(33), 9155–9166.
Hoffman, R. M. (2015). Application of GFP imaging in cancer. Laboratory Investigation, 95(4), 432–452.
Timpson, P., McGhee, E. J., & Anderson, K. I. (2011). Imaging molecular dynamics in vivo–from cell biology to animal models. Journal of Cell Science, 124(Pt 17), 2877–2890.
Naczynski, D. J., et al. (2013). Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nature Communications, 4, 2199.
Kantamneni, H., et al. (2017). Surveillance nanotechnology for multi-organ cancer metastases. Nat Biomed Eng, 1, 993–1003.
Naczynski, D. J., et al. (2010). Albumin nanoshell encapsulation of near-infrared-excitable rare-Earth nanoparticles enhances biocompatibility and enables targeted cell imaging. Small (Weinheim an der Bergstrasse, Germany), 6(15), 1631–1640.
Cui, M., et al. (2013). Multifunctional albumin nanoparticles as combination drug carriers for intra-tumoral chemotherapy. Adv Healthc Mater, 2(9), 1236–1245.
Bennett, Z. T., et al. (2020). Detection of Lymph Node Metastases by Ultra-pH-Sensitive Polymeric Nanoparticles. Theranostics, 10(7), 3340–3350.
Zheng, X., et al. (2015). Tracking Cancer Metastasis In Vivo by Using an Iridium-Based Hypoxia-Activated Optical Oxygen Nanosensor. Angewandte Chemie (International ed. in English), 54(28), 8094–8099.
Molaabasi, F., et al. (2020). Fluorescent Nanoclusters for Imaging of Cells/Stem Cells. Methods in Molecular Biology, 2125, 27–37.
Doolittle, E., et al. (2015). Spatiotemporal Targeting of a Dual-Ligand Nanoparticle to Cancer Metastasis. ACS Nano, 9(8), 8012–8021.
Verry, C., et al., Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci Adv, 2020. 6(29): p. eaay5279.
Venditto, V. J., & Szoka, F. C., Jr. (2013). Cancer nanomedicines: So many papers and so few drugs! Advanced Drug Delivery Reviews, 65(1), 80–88.
Park, K. (2019). The beginning of the end of the nanomedicine hype. Journal of Controlled Release, 305, 221–222.
Anselmo, A. C., & Mitragotri, S. (2016). Nanoparticles in the clinic. Bioeng Transl Med, 1(1), 10–29.
D’Mello, S. R., et al. (2017). The evolving landscape of drug products containing nanomaterials in the United States. Nature Nanotechnology, 12(6), 523–529.
Gradishar, W. J., et al. (2005). Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. Journal of Clinical Oncology, 23(31), 7794–7803.
Gabizon, A., et al. (1994). Clinical studies of liposome-encapsulated doxorubicin. Acta Oncologica, 33(7), 779–786.
Nanobiotix, Nanobiotix Announces First Ever Radioenhancer to Receive European Market Approval. 2019.
Biospace. MagForce Nanotechnologies Receives European Regulatory Approval for Its Nano-Cancer(R) Therapy. 2010; Available from: https://www.biospace.com/article/releases/magforce-nanotechnologies-receives-european-regulatory-approval-for-its-nano-cancer-r-therapy-/?s=74.
de Lazaro, I., & Mooney, D. J. (2021). Obstacles and opportunities in a forward vision for cancer nanomedicine. Nature Materials, 20(11), 1469–1479.
Kemp, J. A., & Kwon, Y. J. (2021). Cancer nanotechnology: Current status and perspectives. Nano Converg, 8(1), 34.
Ventola, C. L. (2017). Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T, 42(12), 742–755.
Russell, L.M., C.H. Liu, and P. Grodzinski, Nanomaterials innovation as an enabler for effective cancer interventions. Biomaterials, 2020. 242: p. 119926.
Pharmaceuticals, A., Alnylam Receives Approval of ONPATTRO™ (patisiran) in Europe. 2018.
Grodzinski, P., et al. (2019). Integrating Nanotechnology into Cancer Care. ACS Nano, 13(7), 7370–7376.
Hare, J. I., et al. (2017). Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Advanced Drug Delivery Reviews, 108, 25–38.
Hartshorn, C. M., et al. (2018). Nanotechnology Strategies To Advance Outcomes in Clinical Cancer Care. ACS Nano, 12(1), 24–43.
Schoenmaker, L., et al., mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm, 2021. 601: p. 120586.
Saxena, M., & Christofori, G. (2013). Rebuilding cancer metastasis in the mouse. Molecular Oncology, 7(2), 283–296.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Avula, L.R., Grodzinski, P. Nanotechnology-aided advancement in the combating of cancer metastasis. Cancer Metastasis Rev 41, 383–404 (2022). https://doi.org/10.1007/s10555-022-10025-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10025-7