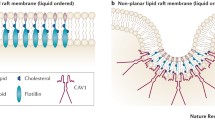
Abstract
Caveolae are specialised and dynamic plasma membrane subdomains, involved in many cellular functions including endocytosis, signal transduction, mechanosensing and lipid storage, trafficking, and metabolism. Two protein families are indispensable for caveola formation and function, namely caveolins and cavins. Mutations of genes encoding these caveolar proteins cause serious pathological conditions such as cardiomyopathies, skeletal muscle diseases, and lipodystrophies. Deregulation of caveola-forming protein expression is associated with many types of cancers including prostate cancer. The distinct function of secretion of the prostatic fluid, and the unique metabolic phenotype of prostate cells relying on lipid metabolism as a main bioenergetic pathway further suggest a significant role of caveolae and caveolar proteins in prostate malignancy. Accumulating in vitro, in vivo, and clinical evidence showed the association of caveolin-1 with prostate cancer grade, stage, metastasis, and drug resistance. In contrast, cavin-1 was found to exhibit tumour suppressive roles. Studies on prostate cancer were the first to show the distinct function of the caveolar proteins depending on their localisation within the caveolar compartment or as cytoplasmic or secreted proteins. In this review, we summarise the roles of caveola-forming proteins in prostate cancer and the potential of exploiting them as therapeutic targets or biological markers.



Similar content being viewed by others
References
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. https://doi.org/10.3322/caac.21492.
Palade, G. (1953). The fine structure of blood capillaries. Journal of Applied Physics, 24, 1424.
Parton, R. G., & Collins, B. M. (2016). Unraveling the architecture of caveolae. Proceedings of the National Academy of Sciences of the United States of America, 113(50), 14170–14172. https://doi.org/10.1073/pnas.1617954113.
Parton, R. G., del Pozo, M. A., Vassilopoulos, S., Nabi, I. R., Le Lay, S., Lundmark, R., et al. (2020). Caveolae: the FAQs. Traffic, 21, 181-185. https://doi.org/10.1111/tra.12689.
Parton, R. G. (2018). Caveolae: structure, function, and relationship to disease. Annual Review of Cell and Developmental Biology, 34(1), 111–136. https://doi.org/10.1146/annurev-cellbio-100617-062737.
Nassar, Z. D., & Parat, M. O. (2015). Cavin family: new players in the biology of caveolae. International Review of Cell and Molecular Biology, 320, 235–305. https://doi.org/10.1016/bs.ircmb.2015.07.009.
Hill, M. M., Bastiani, M., Luetterforst, R., Kirkham, M., Kirkham, A., Nixon, S. J., et al. (2008). PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell, 132(1), 113–124. https://doi.org/10.1016/j.cell.2007.11.042.
Liu, L., Brown, D., McKee, M., Lebrasseur, N. K., Yang, D., Albrecht, K. H., et al. (2008). Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metabolism, 8(4), 310–317. https://doi.org/10.1016/j.cmet.2008.07.008.
Drab, M., Verkade, P., Elger, M., Kasper, M., Lohn, M., Lauterbach, B., et al. (2001). Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science, 293(5539), 2449–2452. https://doi.org/10.1126/science.1062688.
Minetti, C., Bado, M., Broda, P., Sotgia, F., Bruno, C., Galbiati, F., et al. (2002). Impairment of caveolae formation and T-system disorganization in human muscular dystrophy with caveolin-3 deficiency. The American Journal of Pathology, 160(1), 265–270. https://doi.org/10.1016/s0002-9440(10)64370-2.
Nassar, Z. D., Hill, M. M., Parton, R. G., & Parat, M. O. (2013). Caveola-forming proteins caveolin-1 and PTRF in prostate cancer. Nature Reviews. Urology, 10(9), 529–536. https://doi.org/10.1038/nrurol.2013.168.
Senju, Y., Itoh, Y., Takano, K., Hamada, S., & Suetsugu, S. (2011). Essential role of PACSIN2/syndapin-II in caveolae membrane sculpting. Journal of Cell Science, 124(Pt 12), 2032–2040. https://doi.org/10.1242/jcs.086264.
Hansen, C. G., Howard, G., & Nichols, B. J. (2011). Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. Journal of Cell Science, 124(Pt 16), 2777–2785. https://doi.org/10.1242/jcs.084319.
Ludwig, A., Howard, G., Mendoza-Topaz, C., Deerinck, T., Mackey, M., Sandin, S., et al. (2013). Molecular composition and ultrastructure of the caveolar coat complex. PLoS Biology, 11(8), e1001640. https://doi.org/10.1371/journal.pbio.1001640.
Yamaguchi, T., Lu, C., Ida, L., Yanagisawa, K., Usukura, J., Cheng, J., et al. (2016). ROR1 sustains caveolae and survival signalling as a scaffold of cavin-1 and caveolin-1. Nature Communications, 7, 10060. https://doi.org/10.1038/ncomms10060.
Parton, R. G., Tillu, V. A., & Collins, B. M. (2018). Caveolae. Current Biology, 28(8), R402–r405. https://doi.org/10.1016/j.cub.2017.11.075.
Bastiani, M., Liu, L., Hill, M. M., Jedrychowski, M. P., Nixon, S. J., Lo, H. P., et al. (2009). MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. The Journal of Cell Biology, 185(7), 1259–1273. https://doi.org/10.1083/jcb.200903053.
McMahon, K.-A., Zajicek, H., Li, W.-P., Peyton, M. J., Minna, J. D., Hernandez, V. J., et al. (2009). SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. The EMBO Journal, 28(8), 1001–1015. https://doi.org/10.1038/emboj.2009.46.
Hansen, C. G., Bright, N. A., Howard, G., & Nichols, B. J. (2009). SDPR induces membrane curvature and functions in the formation of caveolae. Nature Cell Biology, 11(7), 807–814. https://doi.org/10.1038/ncb1887.
Hommelgaard, A. M., Roepstorff, K., Vilhardt, F., Torgersen, M. L., Sandvig, K., & van Deurs, B. (2005). Caveolae: stable membrane domains with a potential for internalization. Traffic, 6(9), 720–724. https://doi.org/10.1111/j.1600-0854.2005.00314.x.
Ross, S. A., Everson, W. V., & Smart, E. J. (2008). The Pharmacology of caveolae. In C. Ehrhardt & K.-J. Kim (Eds.), Drug absorption studies: in situ, in vitro and in silico models (pp. 598–615). Boston, MA: Springer US.
Spisni, E., Bianco, M., Griffoni, C., Toni, M., D'Angelo, R., Santi, S., et al. (2003). Mechanosensing role of caveolae and caveolar constituents in human endothelial cells. Journal of Cellular Physiology, 197, 198–204. https://doi.org/10.1002/jcp.10344.
Lo, H. P., Hall, T. E., & Parton, R. G. (2016). Mechanoprotection by skeletal muscle caveolae. Bioarchitecture, 6(1), 22–27. https://doi.org/10.1080/19490992.2015.1131891.
Fernandez-Rojo, M. A., & Ramm, G. A. (2016). Caveolin-1 function in liver physiology and disease. Trends in Molecular Medicine, 22(10), 889–904. https://doi.org/10.1016/j.molmed.2016.08.007.
Li, J., Scherl, A., Medina, F., Frank, P. G., Kitsis, R. N., Tanowitz, H. B., et al. (2005). Impaired phagocytosis in caveolin-1 deficient macrophages. Cell Cycle, 4(11), 1599–1607. https://doi.org/10.4161/cc.4.11.2117.
Gupta, R., Toufaily, C., & Annabi, B. (2014). Caveolin and cavin family members: dual roles in cancer. Biochimie, 107(Pt B), 188–202. https://doi.org/10.1016/j.biochi.2014.09.010.
Sotgia, F., Martinez-Outschoorn, U. E., Howell, A., Pestell, R. G., Pavlides, S., & Lisanti, M. P. (2012). Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annual Review of Pathology, 7, 423–467. https://doi.org/10.1146/annurev-pathol-011811-120856.
Gallardo-Arrieta, F., Mogas, T., Magan, L., Garcia, M. A., Garcia, F., Abal, M., et al. (2006). Ultrastructural changes in prostate cells during hormone-induced canine prostatic hyperplasia. Ultrastructural Pathology, 30(6), 435–442. https://doi.org/10.1080/01913120600854079.
Gobbo, M. G., Taboga, S. R., Ribeiro, D. L., & Goes, R. M. (2012). Short-term stromal alterations in the rat ventral prostate following alloxan-induced diabetes and the influence of insulin replacement. Micron, 43(2-3), 326–333. https://doi.org/10.1016/j.micron.2011.09.009.
Gould, M. L., Williams, G., & Nicholson, H. D. (2010). Changes in caveolae, caveolin, and polymerase 1 and transcript release factor (PTRF) expression in prostate cancer progression. The Prostate, 70(15), 1609–1621. https://doi.org/10.1002/pros.21195.
Bai, L., Deng, X., Li, Q., Wang, M., An, W., Deli, A., et al. (2012). Down-regulation of the cavin family proteins in breast cancer. Journal of Cellular Biochemistry, 113(1), 322–328. https://doi.org/10.1002/jcb.23358.
Li, X., Jia, Z., Shen, Y., Ichikawa, H., Jarvik, J., Nagele, R. G., et al. (2008). Coordinate suppression of Sdpr and Fhl1 expression in tumors of the breast, kidney, and prostate. Cancer Science, 99(7), 1326–1333. https://doi.org/10.1111/j.1349-7006.2008.00816.x.
Tian, Y., Yu, Y., Hou, L. K., Chi, J. R., Mao, J. F., Xia, L., et al. (2016). Serum deprivation response inhibits breast cancer progression by blocking transforming growth factor-beta signaling. Cancer Science, 107(3), 274–280. https://doi.org/10.1111/cas.12879.
Ozturk, S., Papageorgis, P., Wong, C. K., Lambert, A. W., Abdolmaleky, H. M., Thiagalingam, A., et al. (2016). SDPR functions as a metastasis suppressor in breast cancer by promoting apoptosis. Proceedings of the National Academy of Sciences of the United States of America, 113(3), 638–643. https://doi.org/10.1073/pnas.1514663113.
Shi, Y., Liu, X., Sun, Y., Wu, D., Qiu, A., Cheng, H., et al. (2015). Decreased expression and prognostic role of EHD2 in human breast carcinoma: correlation with E-cadherin. Journal of Molecular Histology, 46(2), 221–231. https://doi.org/10.1007/s10735-015-9614-7.
Yang, X., Ren, H., Yao, L., Chen, X., & He, A. (2015). Role of EHD2 in migration and invasion of human breast cancer cells. Tumour Biology, 36(5), 3717–3726. https://doi.org/10.1007/s13277-014-3011-9.
Zhang, S., Chen, L., Cui, B., Chuang, H. Y., Yu, J., Wang-Rodriguez, J., et al. (2012). ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One, 7(3), e31127. https://doi.org/10.1371/journal.pone.0031127.
Chien, H. P., Ueng, S. H., Chen, S. C., Chang, Y. S., Lin, Y. C., Lo, Y. F., et al. (2016). Expression of ROR1 has prognostic significance in triple negative breast cancer. Virchows Archiv, 468(5), 589–595. https://doi.org/10.1007/s00428-016-1911-3.
Zhang, S., Zhang, H., Ghia, E. M., Huang, J., Wu, L., Zhang, J., et al. (2019). Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proceedings of the National Academy of Sciences of the United States of America, 116(4), 1370–1377. https://doi.org/10.1073/pnas.1816262116.
Zhang, H., Qiu, J., Ye, C., Yang, D., Gao, L., Su, Y., et al. (2014). ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Scientific Reports, 4, 5811. https://doi.org/10.1038/srep05811.
Yin, Z., Gao, M., Chu, S., Su, Y., Ye, C., Wang, Y., et al. (2017). Antitumor activity of a newly developed monoclonal antibody against ROR1 in ovarian cancer cells. Oncotarget, 8(55), 94210–94222. https://doi.org/10.18632/oncotarget.21618.
Henry, C., Llamosas, E., Knipprath-Meszaros, A., Schoetzau, A., Obermann, E., Fuenfschilling, M., et al. (2015). Targeting the ROR1 and ROR2 receptors in epithelial ovarian cancer inhibits cell migration and invasion. Oncotarget, 6(37), 40310–40326. https://doi.org/10.18632/oncotarget.5643.
Henry, C. E., Llamosas, E., Djordjevic, A., Hacker, N. F., & Ford, C. E. (2016). Migration and invasion is inhibited by silencing ROR1 and ROR2 in chemoresistant ovarian cancer. Oncogenesis, 5(5), e226. https://doi.org/10.1038/oncsis.2016.32.
Zhang, S., Cui, B., Lai, H., Liu, G., Ghia, E. M., Widhopf 2nd, G. F., et al. (2014). Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proceedings of the National Academy of Sciences of the United States of America, 111(48), 17266–17271. https://doi.org/10.1073/pnas.1419599111.
Henry, C. E., Llamosas, E., Daniels, B., Coopes, A., Tang, K., & Ford, C. E. (2018). ROR1 and ROR2 play distinct and opposing roles in endometrial cancer. Gynecologic Oncology, 148(3), 576–584. https://doi.org/10.1016/j.ygyno.2018.01.025.
Wang, X., Liu, Z., & Yang, Z. (2018). Expression and clinical significance of Caveolin-1 in prostate cancer after transurethral surgery. BMC Urology, 18(1), 102. https://doi.org/10.1186/s12894-018-0418-4.
Karam, J. A., Lotan, Y., Roehrborn, C. G., Ashfaq, R., Karakiewicz, P. I., & Shariat, S. F. (2007). Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. The Prostate, 67(6), 614–622. https://doi.org/10.1002/pros.20557.
Goto, T., Nguyen, B. P., Nakano, M., Ehara, H., Yamamoto, N., & Deguchi, T. (2008). Utility of Bcl-2, P53, Ki-67, and caveolin-1 immunostaining in the prediction of biochemical failure after radical prostatectomy in a Japanese population. Urology, 72(1), 167–171. https://doi.org/10.1016/j.urology.2007.11.003.
Panic, A., Ketteler, J., Reis, H., Sak, A., Herskind, C., Maier, P., et al. (2017). Progression-related loss of stromal caveolin 1 levels fosters the growth of human PC3 xenografts and mediates radiation resistance. Scientific Reports, 7(1), 41138. https://doi.org/10.1038/srep41138.
Klein, D., Schmitz, T., Verhelst, V., Panic, A., Schenck, M., Reis, H., et al. (2015). Endothelial caveolin-1 regulates the radiation response of epithelial prostate tumors. Oncogenesis, 4(5), e148–e148. https://doi.org/10.1038/oncsis.2015.9.
Pellinen, T., Blom, S., Sánchez, S., Välimäki, K., Mpindi, J.-P., Azegrouz, H., et al. (2018). ITGB1-dependent upregulation of caveolin-1 switches TGFβ signalling from tumour-suppressive to oncogenic in prostate cancer. Scientific Reports, 8(1), 2338. https://doi.org/10.1038/s41598-018-20161-2.
Yang, G., Goltsov, A. A., Ren, C., Kurosaka, S., Edamura, K., Logothetis, R., et al. (2012). Caveolin-1 upregulation contributes to c-Myc-induced high-grade prostatic intraepithelial neoplasia and prostate cancer. Molecular Cancer Research, 10(2), 218–229. https://doi.org/10.1158/1541-7786.MCR-11-0451.
Zenklusen, J. C., Thompson, J. C., Troncoso, P., Kagan, J., & Conti, C. J. (1994). Loss of heterozygosity in human primary prostate carcinomas: a possible tumor suppressor gene at 7q31. 1. Cancer Research, 54(24), 6370.
Takahashi, S., Qian, J., Brown, J. A., Alcaraz, A., Bostwick, D. G., Lieber, M. M., et al. (1994). Potential markers of prostate cancer aggressiveness detected by fluorescence in situ hybridization in needle biopsies. Cancer Research, 54(13), 3574.
Takahashi, S., Shan, A. L., Ritland, S. R., Delacey, K. A., Bostwick, D. G., Lieber, M. M., et al. (1995). Frequent loss of heterozygosity at 7q31. 1 in primary prostate cancer is associated with tumor aggressiveness and progression. Cancer Research, 55(18), 4114.
Engelman, J. A., Zhang, X. L., Galbiati, F., & Lisanti, M. P. (1998). Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31). FEBS Letters, 429(3), 330–336. https://doi.org/10.1016/s0014-5793(98)00619-x.
Cher, M. L., Bova, G. S., Moore, D. H., Small, E. J., Carroll, P. R., Pin, S. S., et al. (1996). Genetic alterations in untreated metastases and androgen-independent prostate cancer detected by comparative genomic hybridization and allelotyping. Cancer Research, 56(13), 3091.
Joos, S., Bergerheim, U. S. R., Pan, Y., Matsuyama, H., Bentz, M., Manoir, S. D., et al. (1995). Mapping of chromosomal gains and losses in prostate cancer by comparative genomic hybridization. Genes, Chromosomes and Cancer, 14(4), 267–276.
Visakorpi, T., Kallioniemi, A. H., Syvänen, A. C., Hyytinen, E. R., Karhu, R., Tammela, T., et al. (1995). Genetic changes in primary and recurrent prostate cancer by comparative genomic hybridization. Cancer Research, 55(2), 342–347.
Brookman-Amissah, N., Duchesnes, C., Williamson, M., Wang, Q., Ahmed, A., Feneley, M., et al. (2005). Genome-wide screening for genetic changes in a matched pair of benign and prostate cancer cell lines using array CGH. Prostate Cancer and Prostatic Diseases, 8(4), 335–343.
Nupponen, N. N., Kakkola, L., Koivisto, P., & Visakorpi, T. (1998). Genetic alterations in hormone-refractory recurrent prostate carcinomas. The American Journal of Pathology, 153(1), 141–148. https://doi.org/10.1016/S0002-9440(10)65554-X.
Bandyk, M. G., Pisters, L. L., Von Eshenbach, A. C., Chung, L. W. K., Zhao, L., Liang, J. C., et al. (1994). Trisomy 7: a potential cytogenetic marker of human prostate cancer progression. Genes, Chromosomes and Cancer, 9(1), 19–27.
Zitzelsberger, H., Szücs, S., Weier, H. U., Lehmann, L., Braselmann, H., Enders, S., et al. (1994). Numerical abnormalities of chromosome 7 in human prostate cancer detected by fluorescence in situ hybridization (FISH) on paraffin-embedded tissue sections with centromere-specific dna probes. The Journal of Pathology, 172(4), 325–335.
Das, K., Lau, W., Sivaswaren, C., Ph, T., Fook-Chong, S., Sl, T., et al. (2005). Chromosomal changes in prostate cancer: a fluorescence in situ hybridization study. Clinical Genetics, 68(1), 40–47. https://doi.org/10.1111/j.1399-0004.2005.00452.x.
Alcaraz, A., Takahashi, S., Brown, J. A., Herath, J. F., Bergstralh, E. J., Larson-Keller, J. J., et al. (1994). Aneuploidy and aneusomy of chromosome 7 detected by fluorescence in situ hybridization are markers of poor prognosis in prostate cancer. Cancer Research, 54(15), 3998–4002.
Konig, J. J., Teubel, W., Romijn, J. C., Schroder, F. H., & Hagemeijer, A. (1996). Gain and loss of chromosomes 1, 7, 8, 10, 18, and Y in 46 prostate cancers. Human Pathology, 27(7), 720–727.
Cohen, A. W., Hnasko, R., Schubert, W., & Lisanti, M. P. (2004). Role of caveolae and caveolins in health and disease. Physiological Reviews, 84(4), 1341–1379.
Haeusler, J., Hoegel, J., Bachmann, N., Herkommer, K., Paiss, T., Vogel, W., et al. (2005). Association of a CAV-1 haplotype to familial aggressive prostate cancer. The Prostate, 65(2), 171–177.
Cui, J., Rohr, L. R., Swanson, G., Speights, V., Maxwell, T., & Brothma, A. R. (2001). Hypermethylation of the caveolin-1 gene promoter in prostate cancer. The Prostate, 46(3), 249–256.
Bachmann, N., Haeusler, J., Luedeke, M., Kuefer, R., Perner, S., Assum, G., et al. (2008). Expression changes of CAV1 and EZH2, located on 7q31∼q36, are rarely related to genomic alterations in primary prostate carcinoma. Cancer Genetics and Cytogenetics, 182(2), 103–110. https://doi.org/10.1016/j.cancergencyto.2008.01.006.
Woodson, K., Hanson, J., & Tangrea, J. (2004). A survey of gene-specific methylation in human prostate cancer among black and white men. Cancer Letters, 205(2), 181–188. https://doi.org/10.1016/j.canlet.2003.11.027.
Thompson, T. C., Tahir, S. A., Li, L., Watanabe, M., Naruishi, K., Yang, G., et al. (2010). The role of caveolin-1 in prostate cancer: clinical implications. Prostate Cancer and Prostatic Diseases, 13(1), 6–11. https://doi.org/10.1038/pcan.2009.29.
Tahir, S. A., Yang, G., Ebara, S., Timme, T. L., Satoh, T., Li, L., et al. (2001). Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Research, 61(10), 3882–3885.
Satoh, T., Yang, G., Egawa, S., Addai, J., Frolov, A., Kuwao, S., et al. (2003). Caveolin-1 expression is a predictor of recurrence-free survival in pT2N0 prostate carcinoma diagnosed in Japanese patients. Cancer, 97(5), 1225–1233. https://doi.org/10.1002/cncr.11198.
Yang, G., Timme, T. L., Frolov, A., Wheeler, T. M., & Thompson, T. C. (2005). Combined c-Myc and caveolin-1 expression in human prostate carcinoma predicts prostate carcinoma progression. Cancer, 103(6), 1186–1194. https://doi.org/10.1002/cncr.20905.
Moon, H., Lee, C. S., Inder, K. L., Sharma, S., Choi, E., Black, D. M., et al. (2014). PTRF/cavin-1 neutralizes non-caveolar caveolin-1 microdomains in prostate cancer. Oncogene, 33(27), 3561–3570. https://doi.org/10.1038/onc.2013.315.
Nassar, Z. D., Moon, H., Duong, T., Neo, L., Hill, M. M., Francois, M., et al. (2013). PTRF/Cavin-1 decreases prostate cancer angiogenesis and lymphangiogenesis. Oncotarget, 4(10), 1844–1855. https://doi.org/10.18632/oncotarget.1300.
Sugie, S., Mukai, S., Yamasaki, K., Kamibeppu, T., Tsukino, H., & Kamoto, T. (2015). Significant association of caveolin-1 and caveolin-2 with prostate cancer progression. Cancer Genomics Proteomics, 12(6), 391–396.
Liu, P., Li, W. P., Machleidt, T., & Anderson, R. G. (1999). Identification of caveolin-1 in lipoprotein particles secreted by exocrine cells. Nature Cell Biology, 1(6), 369–375. https://doi.org/10.1038/14067.
Burden, H. P., Holmes, C. H., Persad, R., & Whittington, K. (2005). Prostasomes—their effects on human male reproduction and fertility. Human Reproduction Update, 12(3), 283–292. https://doi.org/10.1093/humupd/dmi052.
Llorente, A., de Marco, M. C., & Alonso, M. A. (2004). Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. Journal of Cell Science, 117(Pt 22), 5343–5351. https://doi.org/10.1242/jcs.01420.
Bartz, R., Zhou, J., Hsieh, J.-T., Ying, Y., Li, W., & Liu, P. (2008). Caveolin-1 secreting LNCaP cells induce tumor growth of caveolin-1 negative LNCaP cells in vivo. International Journal of Cancer, 122(3), 520–525. https://doi.org/10.1002/ijc.23142.
Di Vizio, D., Morello, M., Dudley, A. C., Schow, P. W., Adam, R. M., Morley, S., et al. (2012). Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. The American Journal of Pathology, 181(5), 1573–1584. https://doi.org/10.1016/j.ajpath.2012.07.030.
Lin, C. J., Yun, E. J., Lo, U. G., Tai, Y. L., Deng, S., Hernandez, E., et al. (2019). The paracrine induction of prostate cancer progression by caveolin-1. Cell Death & Disease, 10(11), 834. https://doi.org/10.1038/s41419-019-2066-3.
Tahir, S. A., Ren, C., Timme, T. L., Gdor, Y., Hoogeveen, R., Morrisett, J. D., et al. (2003). Development of an immunoassay for serum caveolin-1 a novel biomarker for prostate cancer. Clinical Cancer Research, 9(10), 3653–3659.
Tahir, S. A., Kurosaka, S., Tanimoto, R., Goltsov, A. A., Park, S., & Thompson, T. C. (2013). Serum caveolin-1, a biomarker of drug response and therapeutic target in prostate cancer models. Cancer Biology & Therapy, 14(2), 117–126. https://doi.org/10.4161/cbt.22633.
Tahir, S. A., Frolov, A., Hayes, T. G., Mims, M. P., Miles, B. J., Lerner, S. P., et al. (2006). Preoperative serum caveolin-1 as a prognostic marker for recurrence in a radical prostatectomy cohort. Clinical Cancer Research, 12(16), 4872–4875.
Sugie, S., Mukai, S., Tsukino, H., Toda, Y., Yamauchi, T., Nishikata, I., et al. (2013). Increased plasma caveolin-1 levels are associated with progression of prostate cancer among Japanese men. Anticancer Research, 33(5), 1893–1897.
Yang, G., Addai, J., Wheeler, T. M., Frolov, A., Miles, B. J., Kadmon, D., et al. (2007). Correlative evidence that prostate cancer cell-derived caveolin-1 mediates angiogenesis. Human Pathology, 38(11), 1688–1695. https://doi.org/10.1016/j.humpath.2007.03.024.
Mathieu, R., Klatte, T., Lucca, I., Mbeutcha, A., Seitz, C., Karakiewicz, P. I., et al. (2016). Prognostic value of caveolin-1 in patients treated with radical prostatectomy: a multicentric validation study. BJU International, 118(2), 243–249. https://doi.org/10.1111/bju.13224.
Yang, G., Truong, L. D., Timme, T. L., Ren, C., Wheeler, T. M., Park, S. H., et al. (1998). Elevated expression of caveolin is associated with prostate and breast cancer. Clinical Cancer Research, 4(8), 1873–1880.
Yang, G., Truong, L. D., Wheeler, T. M., & Thompson, T. C. (1999). Caveolin-1 expression in clinically confined human prostate cancer. Cancer Research, 59(22), 5719.
Liu, J. M., Cheng, S. H., Liu, X. X., Xia, C., Wang, W. W., & Ma, X. L. (2015). Prognostic value of caveolin-1 in genitourinary cancer: a meta-analysis. International Journal of Clinical and Experimental Medicine, 8(11), 20760–20768.
Basourakos, S. P., Davis, J. W., Chapin, B. F., Ward, J. F., Pettaway, C. A., Pisters, L. L., et al. (2018). Baseline and longitudinal plasma caveolin-1 level as a biomarker in active surveillance for early-stage prostate cancer. BJU International, 121(1), 69–76. https://doi.org/10.1111/bju.13963.
Lu, P., Weaver, V. M., & Werb, Z. (2012). The extracellular matrix: a dynamic niche in cancer progression. The Journal of Cell Biology, 196(4), 395–406. https://doi.org/10.1083/jcb.201102147.
Provenzano, P. P., Eliceiri, K. W., Campbell, J. M., Inman, D. R., White, J. G., & Keely, P. J. (2006). Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Medicine, 4(1), 38. https://doi.org/10.1186/1741-7015-4-38.
Levental, K. R., Yu, H., Kass, L., Lakins, J. N., Egeblad, M., Erler, J. T., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell, 139(5), 891–906. https://doi.org/10.1016/j.cell.2009.10.027.
Egeblad, M., Nakasone, E. S., & Werb, Z. (2010). Tumors as organs: complex tissues that interface with the entire organism. Developmental Cell, 18(6), 884–901. https://doi.org/10.1016/j.devcel.2010.05.012.
Bremnes, R. M., Donnem, T., Al-Saad, S., Al-Shibli, K., Andersen, S., Sirera, R., et al. (2011). The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. Journal of Thoracic Oncology, 6(1), 209–217. https://doi.org/10.1097/JTO.0b013e3181f8a1bd.
Di Vizio, D., Morello, M., Sotgia, F., Pestell, R. G., Freeman, M. R., & Lisanti, M. P. (2009). An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle, 8(15), 2420–2424. https://doi.org/10.4161/cc.8.15.9116.
Giatromanolaki, A., Koukourakis, M. I., Koutsopoulos, A., Mendrinos, S., & Sivridis, E. (2012). The metabolic interactions between tumor cells and tumor-associated stroma (TAS) in prostatic cancer. Cancer Biology & Therapy, 13(13), 1284–1289. https://doi.org/10.4161/cbt.21785.
Panic, A., Ketteler, J., Reis, H., Sak, A., Herskind, C., Maier, P., et al. (2017). Progression-related loss of stromal caveolin 1 levels fosters the growth of human PC3 xenografts and mediates radiation resistance. Scientific Reports, 7, 41138. https://doi.org/10.1038/srep41138.
Zhu, J., Pan, C., Jiang, J., Deng, M., Gao, H., Men, B., et al. (2015). Six stroma-based RNA markers diagnostic for prostate cancer in European-Americans validated at the RNA and protein levels in patients in China. Oncotarget, 6(18), 16757–16765. https://doi.org/10.18632/oncotarget.4430.
Ayala, G., Morello, M., Frolov, A., You, S., Li, R., Rosati, F., et al. (2013). Loss of caveolin-1 in prostate cancer stroma correlates with reduced relapse-free survival and is functionally relevant to tumour progression. The Journal of Pathology, 231(1), 77–87. https://doi.org/10.1002/path.4217.
Hammarsten, P., Dahl Scherdin, T., Hagglof, C., Andersson, P., Wikstrom, P., Stattin, P., et al. (2016). High caveolin-1 expression in tumor stroma is associated with a favourable outcome in prostate cancer patients managed by watchful waiting. PLoS One, 11(10), e0164016. https://doi.org/10.1371/journal.pone.0164016.
Martinez-Outschoorn, U. E., Balliet, R. M., Rivadeneira, D. B., Chiavarina, B., Pavlides, S., Wang, C., et al. (2010). Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle, 9(16), 3256–3276. https://doi.org/10.4161/cc.9.16.12553.
Martinez-Outschoorn, U. E., Lin, Z., Trimmer, C., Flomenberg, N., Wang, C., Pavlides, S., et al. (2011). Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: implications for PET imaging of human tumors. Cell Cycle, 10(15), 2504–2520. https://doi.org/10.4161/cc.10.15.16585.
Martinez-Outschoorn, U. E., Lisanti, M. P., & Sotgia, F. (2014). Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Seminars in Cancer Biology, 25, 47–60. https://doi.org/10.1016/j.semcancer.2014.01.005.
Ketteler, J., Panic, A., Reis, H., Wittka, A., Maier, P., Herskind, C., et al. (2019). Progression-related loss of stromal caveolin 1 levels mediates radiation resistance in prostate carcinoma via the apoptosis inhibitor TRIAP1. Journal of Clinical Medicine, 8(3). https://doi.org/10.3390/jcm8030348.
Mostaghel, E. A. (2013). Steroid hormone synthetic pathways in prostate cancer. Translational Andrology and Urology, 2(3), 212–227. https://doi.org/10.3978/j.issn.2223-4683.2013.09.16.
Lu, M. L., Schneider, M. C., Zheng, Y., Zhang, X., & Richie, J. P. (2001). Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. The Journal of Biological Chemistry, 276(16), 13442–13451. https://doi.org/10.1074/jbc.M006598200.
Bennett, N. C., Hooper, J. D., Johnson, D. W., & Gobe, G. C. (2014). Expression profiles and functional associations of endogenous androgen receptor and caveolin-1 in prostate cancer cell lines. Prostate, 74(5), 478–487. https://doi.org/10.1002/pros.22767.
Pflug, B. R., Reiter, R. E., & Nelson, J. B. (1999). Caveolin expression is decreased following androgen deprivation in human prostate cancer cell lines. Prostate, 40(4), 269–273. https://doi.org/10.1002/(sici)1097-0045(19990901)40:4<269::aid-pros9>3.0.co;2-6.
Li, L., Yang, G., Ebara, S., Satoh, T., Nasu, Y., Timme, T. L., et al. (2001). Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Research, 61(11), 4386–4392.
Bryant, K. G., Camacho, J., Jasmin, J. F., Wang, C., Addya, S., Casimiro, M. C., et al. (2011). Caveolin-1 overexpression enhances androgen-dependent growth and proliferation in the mouse prostate. The International Journal of Biochemistry & Cell Biology, 43(9), 1318–1329. https://doi.org/10.1016/j.biocel.2011.04.019.
Li, L., Ren, C. H., Tahir, S. A., Ren, C., & Thompson, T. C. (2003). Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Molecular and Cellular Biology, 23(24), 9389–9404. https://doi.org/10.1128/mcb.23.24.9389-9404.2003.
Nasu, Y., Timme, T. L., Yang, G., Bangma, C. H., Li, L., Ren, C., et al. (1998). Suppression of caveolin expression induces androgen sensitivity in metastatic androgen-insensitive mouse prostate cancer cells. Nature Medicine, 4(9), 1062–1064. https://doi.org/10.1038/2048.
Gao, Y., Li, L., Li, T., Ma, L., Yuan, M., Sun, W., et al. (2019). Simvastatin delays castrationresistant prostate cancer metastasis and androgen receptor antagonist resistance by regulating the expression of caveolin1. International Journal of Oncology, 54(6), 2054–2068. https://doi.org/10.3892/ijo.2019.4774.
Mouraviev, V., Li, L., Tahir, S. A., Yang, G., Timme, T. M., Goltsov, A., et al. (2002). The role of caveolin-1 in androgen insensitive prostate cancer. The Journal of Urology, 168(4 Pt 1), 1589–1596. https://doi.org/10.1097/01.ju.0000030164.80605.b9.
Rajan, P., Sudbery, I. M., Villasevil, M. E. M., Mui, E., Fleming, J., Davis, M., et al. (2014). Next-generation sequencing of advanced prostate cancer treated with androgen-deprivation therapy. European Urology, 66(1), 32–39. https://doi.org/10.1016/j.eururo.2013.08.011.
Watanabe, M., Yang, G., Cao, G., Tahir, S. A., Naruishi, K., Tabata, K.-I., et al. (2009). Functional analysis of secreted caveolin-1 in mouse models of prostate cancer progression. Molecular Cancer Research, 7(9), 1446–1455. https://doi.org/10.1158/1541-7786.mcr-09-0071.
Tahir, S. A., Yang, G., Goltsov, A. A., Watanabe, M., Tabata, K., Addai, J., et al. (2008). Tumor cell-secreted caveolin-1 has proangiogenic activities in prostate cancer. Cancer Research, 68(3), 731–739. https://doi.org/10.1158/0008-5472.CAN-07-2668.
Ayala, G. E., Dai, H., Tahir, S. A., Li, R., Timme, T., Ittmann, M., et al. (2006). Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Research, 66(10), 5159–5164. https://doi.org/10.1158/0008-5472.can-05-1847.
Tahir, S. A., Park, S., & Thompson, T. C. (2009). Caveolin-1 regulates VEGF-stimulated angiogenic activities in prostate cancer and endothelial cells. Cancer Biology & Therapy, 8(23), 2286.
Timme, T. L., Goltsov, A., Tahir, S., Likun, L., Jianxiang, W., Chengzhen, R., et al. (2000). Caveolin-1 is regulated by c-myc and suppresses c-myc-induced apoptosis. Oncogene, 19(29), 3256–3265.
Williams, T. M., Hassan, G. S., Li, J., Cohen, A. W., Medina, F., Frank, P. G., et al. (2005). Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer. The Journal of Biological Chemistry, 280(26), 25134.
Li, L., Ren, C., Yang, G., Goltsov, A. A., Tabata, K.-I., & Thompson, T. C. (2009). Caveolin-1 promotes autoregulatory, Akt-mediated induction of cancer-promoting growth factors in prostate cancer cells. Molecular Cancer Research, 7(11), 1781–1791. https://doi.org/10.1158/1541-7786.mcr-09-0255.
Zhang, X., Ling, M. T., Wang, Q., Lau, C. K., Leung, S. C., Lee, T. K., et al. (2007). Identification of a novel inhibitor of differentiation-1 (ID-1) binding partner, caveolin-1, and its role in epithelial-mesenchymal transition and resistance to apoptosis in prostate cancer cells. The Journal of Biological Chemistry, 282(46), 33284–33294. https://doi.org/10.1074/jbc.M705089200.
Kamibeppu, T., Yamasaki, K., Nakahara, K., Nagai, T., Terada, N., Tsukino, H., et al. (2018). Caveolin-1 and -2 regulate cell motility in castration-resistant prostate cancer. Research and Reports in Urology, 10, 135–144. https://doi.org/10.2147/rru.S173377.
Nassar, Z. D., Hill, M. M., Parton, R. G., Francois, M., & Parat, M. O. (2015). Non-caveolar caveolin-1 expression in prostate cancer cells promotes lymphangiogenesis. Oncoscience, 2(7), 635–645.
Aboulaich, N., Chui, P. C., Asara, J. M., Flier, J. S., & Maratos-Flier, E. (2011). Polymerase I and transcript release factor regulates lipolysis via a phosphorylation-dependent mechanism. Diabetes, 60(3), 757–765. https://doi.org/10.2337/db10-0744.
Pol, A., Martin, S., Fernandez, M. A., Ferguson, C., Carozzi, A., Luetterforst, R., et al. (2003). Dynamic and regulated association of caveolin with lipid bodies: modulation of lipid body motility and function by a dominant negative mutant. Molecular Biology of the Cell, 15(1), 99–110. https://doi.org/10.1091/mbc.e03-06-0368.
Aboulaich, N., Örtegren, U., Vener, A. V., & Strålfors, P. (2006). Association and insulin regulated translocation of hormone-sensitive lipase with PTRF. Biochemical and Biophysical Research Communications, 350(3), 657–661. https://doi.org/10.1016/j.bbrc.2006.09.094.
Cao, H., Alston, L., Ruschman, J., & Hegele, R. A. (2008). Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids in Health and Disease, 7, 3. https://doi.org/10.1186/1476-511x-7-3.
Kim, C. A., Delepine, M., Boutet, E., El Mourabit, H., Le Lay, S., Meier, M., et al. (2008). Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. The Journal of Clinical Endocrinology and Metabolism, 93(4), 1129–1134. https://doi.org/10.1210/jc.2007-1328.
Shastry, S., Delgado, M. R., Dirik, E., Turkmen, M., Agarwal, A. K., & Garg, A. (2010). Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. American Journal of Medical Genetics. Part A, 152a(9), 2245–2253. https://doi.org/10.1002/ajmg.a.33578.
Hayashi, Y. K., Matsuda, C., Ogawa, M., Goto, K., Tominaga, K., Mitsuhashi, S., et al. (2009). Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. The Journal of Clinical Investigation, 119(9), 2623–2633. https://doi.org/10.1172/jci38660.
Asterholm, I. W., Mundy, D. I., Weng, J., Anderson, R. G., & Scherer, P. E. (2012). Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metabolism, 15(2), 171–185. https://doi.org/10.1016/j.cmet.2012.01.004.
Fernandez-Rojo, M. A., Gongora, M., Fitzsimmons, R. L., Martel, N., Martin, S. D., Nixon, S. J., et al. (2013). Caveolin-1 is necessary for hepatic oxidative lipid metabolism: evidence for crosstalk between caveolin-1 and bile acid signaling. Cell Reports, 4(2), 238–247. https://doi.org/10.1016/j.celrep.2013.06.017.
Fridolfsson, H. N., Kawaraguchi, Y., Ali, S. S., Panneerselvam, M., Niesman, I. R., Finley, J. C., et al. (2012). Mitochondria-localized caveolin in adaptation to cellular stress and injury. The FASEB Journal, 26(11), 4637–4649. https://doi.org/10.1096/fj.12-215798.
Liu, Y. (2006). Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer and Prostatic Diseases, 9(3), 230–234. https://doi.org/10.1038/sj.pcan.4500879.
Mah, C. Y., Nassar, Z. D., Swinnen, J. V., & Butler, L. M. (2019). Lipogenic effects of androgen signaling in normal and malignant prostate. Asian Journal of Urology. https://doi.org/10.1016/j.ajur.2019.12.003.
Zadra, G., Photopoulos, C., & Loda, M. (2013). The fat side of prostate cancer. Biochimica et Biophysica Acta, 1831(10), 1518–1532. https://doi.org/10.1016/j.bbalip.2013.03.010.
Rossi, S., Graner, E., Febbo, P., Weinstein, L., Bhattacharya, N., Onody, T., et al. (2003). Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Molecular Cancer Research, 1(10), 707–715.
Swinnen, J. V., Vanderhoydonc, F., Elgamal, A. A., Eelen, M., Vercaeren, I., Joniau, S., et al. (2000). Selective activation of the fatty acid synthesis pathway in human prostate cancer. International Journal of Cancer, 88(2), 176–179. https://doi.org/10.1002/1097-0215(20001015)88:2<176::aid-ijc5>3.0.co;2-3.
Shurbaji, M. S., Kalbfleisch, J. H., & Thurmond, T. S. (1996). Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Human Pathology, 27(9), 917–921. https://doi.org/10.1016/s0046-8177(96)90218-x.
Boopathi, E., Gomes, C. M., Goldfarb, R., John, M., Srinivasan, V. G., Alanzi, J., et al. (2011). Transcriptional repression of caveolin-1 (CAV1) gene expression by GATA-6 in bladder smooth muscle hypertrophy in mice and human beings. The American Journal of Pathology, 178(5), 2236–2251. https://doi.org/10.1016/j.ajpath.2011.01.038.
Cao, S., Fernandez-Zapico, M. E., Jin, D., Puri, V., Cook, T. A., Lerman, L. O., et al. (2005). KLF11-mediated repression antagonizes Sp1/sterol-responsive element-binding protein-induced transcriptional activation of caveolin-1 in response to cholesterol signaling. The Journal of Biological Chemistry, 280(3), 1901–1910. https://doi.org/10.1074/jbc.M407941200.
Bist, A., Fielding, P. E., & Fielding, C. J. (1997). Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol. Proceedings of the National Academy of Sciences of the United States of America, 94(20), 10693–10698. https://doi.org/10.1073/pnas.94.20.10693.
Di Vizio, D., Adam, R. M., Kim, J., Kim, R., Sotgia, F., Williams, T., et al. (2008). Caveolin-1 interacts with a lipid raft-associated population of fatty acid synthase. Cell Cycle, 7(14), 2257–2267. https://doi.org/10.4161/cc.7.14.6475.
Di Vizio, D., Sotgia, F., Williams, T. M., Hassan, G. S., Capozza, F., Frank, P. G., et al. (2007). Caveolin-1 is required for the upregulation of fatty acid synthase (FASN), a tumor promoter, during prostate cancer progression. Cancer Biology & Therapy, 6(8), 1269–1274.
Karantanos, T., Karanika, S., Wang, J., Yang, G., Dobashi, M., Park, S., et al. (2016). Caveolin-1 regulates hormone resistance through lipid synthesis, creating novel therapeutic opportunities for castration-resistant prostate cancer. Oncotarget, 7(29), 46321–46334. https://doi.org/10.18632/oncotarget.10113.
Watt, M. J., Clark, A. K., Selth, L. A., Haynes, V. R., Lister, N., Rebello, R., et al. (2019). Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Science Translational Medicine, 11(478). https://doi.org/10.1126/scitranslmed.aau5758.
Mattern, H. M., Raikar, L. S., & Hardin, C. D. (2009). The effect of caveolin-1 (Cav-1) on fatty acid uptake and CD36 localization and lipotoxicity in vascular smooth muscle (VSM) cells. International Journal of Physiology, Pathophysiology and Pharmacology, 1(1), 1–14.
Ring, A., Le Lay, S., Pohl, J., Verkade, P., & Stremmel, W. (2006). Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochimica et Biophysica Acta, 1761(4), 416–423. https://doi.org/10.1016/j.bbalip.2006.03.016.
Meshulam, T., Simard, J. R., Wharton, J., Hamilton, J. A., & Pilch, P. F. (2006). Role of caveolin-1 and cholesterol in transmembrane fatty acid movement. Biochemistry, 45(9), 2882–2893. https://doi.org/10.1021/bi051999b.
Simard, J. R., Meshulam, T., Pillai, B. K., Kirber, M. T., Brunaldi, K., Xu, S., et al. (2010). Caveolins sequester FA on the cytoplasmic leaflet of the plasma membrane, augment triglyceride formation, and protect cells from lipotoxicity. Journal of Lipid Research, 51(5), 914–922. https://doi.org/10.1194/jlr.M900251.
Ding, S.-Y., Lee, M.-J., Summer, R., Liu, L., Fried, S. K., & Pilch, P. F. (2014). Pleiotropic effects of cavin-1 deficiency on lipid metabolism. The Journal of Biological Chemistry, 289(12), 8473–8483. https://doi.org/10.1074/jbc.M113.546242.
Tahir, S. A., Yang, G., Goltsov, A., Song, K.-D., Ren, C., Wang, J., et al. (2013). Caveolin-1-LRP6 signaling module stimulates aerobic glycolysis in prostate cancer. Cancer Research, 73(6), 1900–1911. https://doi.org/10.1158/0008-5472.CAN-12-3040.
Flavin, R., Zadra, G., & Loda, M. (2011). Metabolic alterations and targeted therapies in prostate cancer. The Journal of Pathology, 223(2), 283–294. https://doi.org/10.1002/path.2809.
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 324(5930), 1029–1033. https://doi.org/10.1126/science.1160809.
Jansa, P., Mason, S. W., Hoffmann-Rohrer, U., & Grummt, I. (1998). Cloning and functional characterization of PTRF, a novel protein which induces dissociation of paused ternary transcription complexes. The EMBO Journal, 17(10), 2855–2864. https://doi.org/10.1093/emboj/17.10.2855.
Jansa, P., Burek, C., Sander, E. E., & Grummt, I. (2001). The transcript release factor PTRF augments ribosomal gene transcription by facilitating reinitiation of RNA polymerase I. Nucleic Acids Research, 29(2), 423–429.
Jansa, P., & Grummt, I. (1999). Mechanism of transcription termination: PTRF interacts with the largest subunit of RNA polymerase I and dissociates paused transcription complexes from yeast and mouse. Molecular and General Genetics MGG, 262(3), 508–514.
Vinten, J., Johnsen, A., Roepstorff, P., Harpøth, J., & Tranum-Jensen, J. (2005). Identification of a major protein on the cytosolic face of caveolae. Biochimica et Biophysica Acta, Biomembranes, 1717(1), 34–40.
Liu, L., & Pilch, P. F. (2008). A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. The Journal of Biological Chemistry, 283(7), 4314–4322.
Liu, L., & Pilch, P. F. (2016). PTRF/Cavin-1 promotes efficient ribosomal RNA transcription in response to metabolic challenges. Elife, 5. https://doi.org/10.7554/eLife.17508.
McMahon, K.-A., Wu, Y., Gambin, Y., Sierecki, E., Tillu, V. A., Hall, T., et al. (2019). Identification of intracellular cavin target proteins reveals cavin-PP1alpha interactions regulate apoptosis. Nature Communications, 10(1), 3279. https://doi.org/10.1038/s41467-019-11111-1.
Sinha, B., Koster, D., Ruez, R., Gonnord, P., Bastiani, M., Abankwa, D., et al. (2011). Cells respond to mechanical stress by rapid disassembly of caveolae. Cell, 144(3), 402–413. https://doi.org/10.1016/j.cell.2010.12.031.
Gambin, Y., Ariotti, N., McMahon, K. A., Bastiani, M., Sierecki, E., Kovtun, O., et al. (2013). Single-molecule analysis reveals self assembly and nanoscale segregation of two distinct cavin subcomplexes on caveolae. Elife, 3, e01434. https://doi.org/10.7554/eLife.01434.
Aung, C. S., Hill, M. M., Bastiani, M., Parton, R. G., & Parat, M. O. (2011). PTRF-cavin-1 expression decreases the migration of PC3 prostate cancer cells: role of matrix metalloprotease 9. European Journal of Cell Biology, 90(2-3), 136–142. https://doi.org/10.1016/j.ejcb.2010.06.004.
Hill, M. M., Daud, N. H., Aung, C. S., Loo, D., Martin, S., Murphy, S., et al. (2012). Co-regulation of cell polarization and migration by caveolar proteins PTRF/Cavin-1 and caveolin-1. PLoS One, 7(8), e43041. https://doi.org/10.1371/journal.pone.0043041.
Inder, K. L., Zheng, Y. Z., Davis, M. J., Moon, H., Loo, D., Nguyen, H., et al. (2012). Expression of PTRF in PC-3 cells modulates cholesterol dynamics and the actin cytoskeleton impacting secretion pathways. Molecular & Cellular Proteomics, 11(2), M111.012245. https://doi.org/10.1074/mcp.M111.012245.
Inder, K. L., Ruelcke, J. E., Petelin, L., Moon, H., Choi, E., Rae, J., et al. (2014). Cavin-1/PTRF alters prostate cancer cell-derived extracellular vesicle content and internalization to attenuate extracellular vesicle-mediated osteoclastogenesis and osteoblast proliferation. Journal of Extracellular Vesicles, 3. https://doi.org/10.3402/jev.v3.23784.
Ni, Y., Hao, J., Hou, X., Du, W., Yu, Y., Chen, T., et al. (2018). Dephosphorylated polymerase I and transcript release factor prevents allergic asthma exacerbations by limiting IL-33 release. Frontiers in Immunology, 9(1422). https://doi.org/10.3389/fimmu.2018.01422.
Bai, L., Deng, X., Li, J., Wang, M., Li, Q., An, W., et al. (2011). Regulation of cellular senescence by the essential caveolar component PTRF/Cavin-1. Cell Research, 21(7), 1088–1101. https://doi.org/10.1038/cr.2011.56.
Aboulaich, N., Vainonen, J. P., Stralfors, P., & Vener, A. V. (2004). Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. The Biochemical Journal, 383(Pt 2), 237–248. https://doi.org/10.1042/bj20040647.
Wei, Z., Zou, X., Wang, H., Lei, J., Wu, Y., & Liao, K. (2015). The N-terminal leucine-zipper motif in PTRF/cavin-1 is essential and sufficient for its caveolae-association. Biochemical and Biophysical Research Communications, 456(3), 750–756. https://doi.org/10.1016/j.bbrc.2014.12.035.
Murata, M., Peranen, J., Schreiner, R., Wieland, F., Kurzchalia, T. V., & Simons, K. (1995). VIP21/caveolin is a cholesterol-binding protein. Proceedings of the National Academy of Sciences of the United States of America, 92(22), 10339–10343. https://doi.org/10.1073/pnas.92.22.10339.
Hailstones, D., Sleer, L. S., Parton, R. G., & Stanley, K. K. (1998). Regulation of caveolin and caveolae by cholesterol in MDCK cells. Journal of Lipid Research, 39(2), 369–379.
Rothberg, K. G., Ying, Y. S., Kamen, B. A., & Anderson, R. G. W. (1990). Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. Journal of Cell Biology, 111(6 II), 2931–2938. https://doi.org/10.1083/jcb.111.6.2931.
Breen, M. R., Camps, M., Carvalho-Simoes, F., Zorzano, A., & Pilch, P. F. (2012). Cholesterol depletion in adipocytes causes caveolae collapse concomitant with proteosomal degradation of cavin-2 in a switch-like fashion. PLoS One, 7(4), e34516. https://doi.org/10.1371/journal.pone.0034516.
Kowalska, K., Habrowska-Gorczynska, D. E., Neumayer, C., Bolliger, M., Domenig, C., Piastowska-Ciesielska, A. W., et al. (2018). Lower levels of Caveolin-1 and higher levels of endothelial nitric oxide synthase are observed in abdominal aortic aneurysm patients treated with simvastatin. Acta Biochimica Polonica, 65(1), 111–118. https://doi.org/10.18388/abp.2017_2305.
Moon, H., Ruelcke, J. E., Choi, E., Sharpe, L. J., Nassar, Z. D., Bielefeldt-Ohmann, H., et al. (2015). Diet-induced hypercholesterolemia promotes androgen-independent prostate cancer metastasis via IQGAP1 and caveolin-1. Oncotarget, 6(10), 7438–7453. https://doi.org/10.18632/oncotarget.3476.
Piastowska-Ciesielska, A. W., Kozłowski, M., Wagner, W., Domińska, K., & Ochędalski, T. (2013). Effect of an angiotensin II type 1 receptor blocker on caveolin-1 expression in prostate cancer cells. Archives of Medical Science. AMS, 9(4), 739–744. https://doi.org/10.5114/aoms.2012.30955.
Iguchi, K., Matsunaga, S., Nakano, T., Usui, S., & Hirano, K. (2006). Inhibition of caveolin-1 expression by incadronate in PC-3 prostate cells. Anticancer Research, 26(4b), 2977–2981.
Goh, M., Chen, F., Paulsen, M. T., Yeager, A. M., Dyer, E. S., & Ljungman, M. (2001). Phenylbutyrate attenuates the expression of Bcl-X(L), DNA-PK, caveolin-1, and VEGF in prostate cancer cells. Neoplasia, 3(4), 331–338. https://doi.org/10.1038/sj.neo.7900165.
Guo, Z., Hu, X., Xing, Z., Xing, R., Lv, R., Cheng, X., et al. (2015). Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Molecular and Cellular Biochemistry, 406(1-2), 111–119. https://doi.org/10.1007/s11010-015-2429-8.
Hirsch, G. E., Parisi, M. M., Martins, L. A., Andrade, C. M., Barbe-Tuana, F. M., & Guma, F. T. (2015). gamma-Oryzanol reduces caveolin-1 and PCGEM1 expression, markers of aggressiveness in prostate cancer cell lines. Prostate, 75(8), 783–797. https://doi.org/10.1002/pros.22960.
Yuan, S., Wang, L., Chen, X., Fan, B., Yuan, Q., Zhang, H., et al. (2016). Triptolide inhibits the migration and invasion of human prostate cancer cells via Caveolin-1/CD147/MMPs pathway. Biomedicine & Pharmacotherapy, 84, 1776–1782. https://doi.org/10.1016/j.biopha.2016.10.104.
Palozza, P., Sestito, R., Picci, N., Lanza, P., Monego, G., & Ranelletti, F. O. (2008). The sensitivity to beta-carotene growth-inhibitory and proapoptotic effects is regulated by caveolin-1 expression in human colon and prostate cancer cells. Carcinogenesis, 29(11), 2153–2161. https://doi.org/10.1093/carcin/bgn018.
Markham, A. (2019). Alpelisib: first global approval. Drugs, 79(11), 1249–1253. https://doi.org/10.1007/s40265-019-01161-6.
Cerami, E., Gao, J., Dogrusoz, U., Gross, B. E., Sumer, S. O., Aksoy, B. A., et al. (2012). The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discovery, 2(5), 401–404. https://doi.org/10.1158/2159-8290.cd-12-0095.
Gao, J., Aksoy, B. A., Dogrusoz, U., Dresdner, G., Gross, B., Sumer, S. O., et al. (2013). Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science Signaling, 6(269), l1. https://doi.org/10.1126/scisignal.2004088.
Prensner, J. R., Iyer, M. K., Balbin, O. A., Dhanasekaran, S. M., Cao, Q., Brenner, J. C., et al. (2011). Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nature Biotechnology, 29(8), 742–749. https://doi.org/10.1038/nbt.1914.
Yang, G., Addai, J., Ittmann, M., Wheeler, T. M., & Thompson, T. C. (2000). Elevated caveolin-1 levels in African-American versus white-American prostate cancer. Clinical Cancer Research, 6(9), 3430–3433.
Gumulec, J., Sochor, J., Hlavna, M., Sztalmachova, M., Krizkova, S., Babula, P., et al. (2012). Caveolin-1 as a potential high-risk prostate cancer biomarker. Oncology Reports, 27(3), 831–841. https://doi.org/10.3892/or.2011.1587.
Acknowledgments
The results shown here are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
Funding
ZDN is supported by an Early Career Fellowship from the National Health and Medical Research Council of Australia (1138648), a John Mills Young Investigator Award from the Prostate Cancer Foundation of Australia (YI 1417), and the Cure Cancer Australia Priority-driven Collaborative Cancer Research Scheme (1164798).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nassar, Z.D., Parat, MO. Caveola-forming proteins and prostate cancer. Cancer Metastasis Rev 39, 415–433 (2020). https://doi.org/10.1007/s10555-020-09874-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-020-09874-x