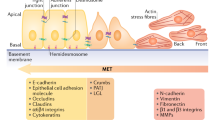
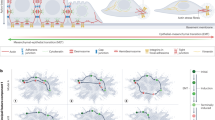
Abstract
Carcinoma cells that are induced to suppress their epithelial features and upregulate mesenchymal gene expression programs acquire traits that promote an invasive and metastatic phenotype. This is achieved through the expression of a program termed the epithelial-to-mesenchymal transition (EMT)—a fundamental cell-biological process that plays key roles in embryogenesis and wound healing. Re-activation of the EMT during cancer promotes disease progression and enhances the metastatic phenotype by bestowing upon previously benign carcinoma cell traits such as migration, invasion, resistance to anoikis, chemoresistance and tumour-initiating potential. Herein, we discuss recent insights into the function of the EMT and cancer cell plasticity during cancer progression, with a focus on their role in promoting successful completion of the later stages of the metastatic cascade.


Similar content being viewed by others
References
Hay, E. D. (2005). The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Developmental Dynamics, 233(3), 706–720.
Lamouille, S., Xu, J., & Derynck, R. (2014). Molecular mechanisms of epithelial-mesenchymal transition. Nature Reviews. Molecular Cell Biology, 15(3), 178–196.
Peinado, H., Olmeda, D., & Cano, A. (2007). Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nature Reviews. Cancer, 7(6), 415–428.
Thiery, J. P., Acloque, H., Huang, R. Y., & Nieto, M. A. (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139(5), 871–890.
Chaffer, C. L., & Weinberg, R. A. (2011). A perspective on cancer cell metastasis. Science, 331(6024), 1559–1564.
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100(7), 3983–3988.
Mani, S. A., Guo, W., Liao, M. J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133(4), 704–715.
Morel, A. P., Lievre, M., Thomas, C., Hinkal, G., Ansieau, S., & Puisieux, A. (2008). Generation of breast cancer stem cells through epithelial-mesenchymal transition. PloS One, 3(8), e2888.
Chen, J., Li, Y., Yu, T. S., McKay, R. M., Burns, D. K., Kernie, S. G., et al. (2012). A restricted cell population propagates glioblastoma growth after chemotherapy. Nature, 488(7412), 522–526.
Kurrey, N. K., Jalgaonkar, S. P., Joglekar, A. V., Ghanate, A. D., Chaskar, P. D., Doiphode, R. Y., et al. (2009). Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells, 27(9), 2059–2068.
Gupta, P. B., Onder, T. T., Jiang, G., Tao, K., Kuperwasser, C., Weinberg, R. A., et al. (2009). Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell, 138(4), 645–659.
Zhang, P., Wei, Y., Wang, L., Debeb, B. G., Yuan, Y., Zhang, J., et al. (2014). ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1. Nature Cell Biology, 16(9), 864–875.
Creighton, C. J., Li, X., Landis, M., Dixon, J. M., Neumeister, V. M., Sjolund, A., et al. (2009). Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proceedings of the National Academy of Sciences of the United States of America, 106(33), 13820–13825.
Smith, B. N., & Bhowmick, N. A. (2016). Role of EMT in metastasis and therapy resistance. J Clin Med, 5(2).
Nieto, M. A., Huang, R. Y., Jackson, R. A., & Thiery, J. P. (2016). EMT: 2016. Cell, 166(1), 21–45.
Bhowmick, N. A., Neilson, E. G., & Moses, H. L. (2004). Stromal fibroblasts in cancer initiation and progression. Nature, 432(7015), 332–337.
Scheel, C., Eaton, E. N., Li, S. H., Chaffer, C. L., Reinhardt, F., Kah, K. J., et al. (2011). Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell, 145(6), 926–940.
Guo, W. (2014). Concise review: breast cancer stem cells: regulatory networks, stem cell niches, and disease relevance. Stem Cells Transl Med, 3(8), 942–948.
Wellner, U., Schubert, J., Burk, U. C., Schmalhofer, O., Zhu, F., Sonntag, A., et al. (2009). The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nature Cell Biology, 11(12), 1487–1495.
Kim, N. H., Kim, H. S., Li, X. Y., Lee, I., Choi, H. S., Kang, S. E., et al. (2011). A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. The Journal of Cell Biology, 195(3), 417–433.
Siemens, H., Jackstadt, R., Hunten, S., Kaller, M., Menssen, A., Gotz, U., et al. (2011). miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle, 10(24), 4256–4271.
Chaffer, C. L., Marjanovic, N. D., Lee, T., Bell, G., Kleer, C. G., Reinhardt, F., et al. (2013). Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell, 154(1), 61–74.
Siegel, P. M., & Massague, J. (2003). Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nature Reviews. Cancer, 3(11), 807–821.
Oft, M., Peli, J., Rudaz, C., Schwarz, H., Beug, H., & Reichmann, E. (1996). TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes & Development, 10(19), 2462–2477.
Muthusamy, B. P., Budi, E. H., Katsuno, Y., Lee, M. K., Smith, S. M., Mirza, A. M., et al. (2015). ShcA protects against epithelial-mesenchymal transition through compartmentalized inhibition of TGF-beta-induced Smad activation. PLoS Biology, 13(12), e1002325.
Gregory, P. A., Bracken, C. P., Smith, E., Bert, A. G., Wright, J. A., Roslan, S., et al. (2011). An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Molecular Biology of the Cell, 22(10), 1686–1698.
Nieswandt, B., Hafner, M., Echtenacher, B., & Männel, D. N. (1999). Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Research, 59(6), 1295.
Trikha, M., Zhou, Z., Timar, J., Raso, E., Kennel, M., Emmell, E., et al. (2002). Multiple roles for platelet GPIIb/IIIa and alphavbeta3 integrins in tumor growth, angiogenesis, and metastasis. Cancer Research, 62(10), 2824–2833.
Labelle, M., Begum, S., & Hynes, R. O. (2011). Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell, 20(5), 576–590.
Kaplan, R. N., Riba, R. D., Zacharoulis, S., Bramley, A. H., Vincent, L., Costa, C., et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature, 438(7069), 820–827.
Hiratsuka, S., Watanabe, A., Aburatani, H., & Maru, Y. (2006). Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature Cell Biology, 8(12), 1369–1375.
Peinado, H., Lavotshkin, S., & Lyden, D. (2011). The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Seminars in Cancer Biology, 21(2), 139–146.
Castellana, D., Zobairi, F., Martinez, M. C., Panaro, M. A., Mitolo, V., Freyssinet, J. M., et al. (2009). Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Research, 69(3), 785–793.
Canesin, G., Cuevas, E. P., Santos, V., Lopez-Menendez, C., Moreno-Bueno, G., Huang, Y., et al. (2015). Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: novel partners in E-cadherin repression and early metastasis colonization. Oncogene, 34(8), 951–964.
Alix-Panabieres, C., Riethdorf, S., & Pantel, K. (2008). Circulating tumor cells and bone marrow micrometastasis. Clinical Cancer Research, 14(16), 5013–5021.
Gunasinghe, N. P., Wells, A., Thompson, E. W., & Hugo, H. J. (2012). Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Reviews, 31(3–4), 469–478.
Stankic, M., Pavlovic, S., Chin, Y., Brogi, E., Padua, D., Norton, L., et al. (2013). TGF-beta-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Reports, 5(5), 1228–1242.
Livasy, C. A., Karaca, G., Nanda, R., Tretiakova, M. S., Olopade, O. I., Moore, D. T., et al. (2006). Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Modern Pathology, 19(2), 264–271.
Rakha, E. A., Putti, T. C., Abd El-Rehim, D. M., Paish, C., Green, A. R., Powe, D. G., et al. (2006). Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. The Journal of Pathology, 208(4), 495–506.
Bonnomet, A., Syne, L., Brysse, A., Feyereisen, E., Thompson, E. W., Noel, A., et al. (2012). A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene, 31(33), 3741–3753.
Schmidt, J. M., Panzilius, E., Bartsch, H. S., Irmler, M., Beckers, J., Kari, V., et al. (2015). Stem-cell-like properties and epithelial plasticity arise as stable traits after transient Twist1 activation. Cell Reports, 10(2), 131–139.
Tran, H. D., Luitel, K., Kim, M., Zhang, K., Longmore, G. D., & Tran, D. D. (2014). Transient SNAIL1 expression is necessary for metastatic competence in breast cancer. Cancer Research, 74(21), 6330–6340.
Tsai, J. H., Donaher, J. L., Murphy, D. A., Chau, S., & Yang, J. (2012). Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell, 22(6), 725–736.
Chaffer, C. L., Brennan, J. P., Slavin, J. L., Blick, T., Thompson, E. W., & Williams, E. D. (2006). Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Research, 66(23), 11271–11278.
Ye, X., Tam, W. L., Shibue, T., Kaygusuz, Y., Reinhardt, F., Ng Eaton, E., et al. (2015). Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature, 525(7568), 256–260.
Brabletz, S., & Brabletz, T. (2010). The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO Reports, 11(9), 670–677.
Ye, X., & Weinberg, R. A. (2015). Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends in Cell Biology, 25(11), 675–686.
De Craene, B., & Berx, G. (2013). Regulatory networks defining EMT during cancer initiation and progression. Nature Reviews. Cancer, 13(2), 97–110.
De Cock, J. M., Shibue, T., Dongre, A., Keckesova, Z., Reinhardt, F., & Weinberg, R. A. (2016). Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Res.
Zhao, Z., Zhu, X., Cui, K., Mancuso, J., Federley, R., Fischer, K., et al. (2016). In vivo visualization and characterization of epithelial-mesenchymal transition in breast tumors. Cancer Research, 76(8), 2094–2104.
Cortez, M. A., Valdecanas, D., Zhang, X., Zhan, Y., Bhardwaj, V., Calin, G. A., et al. (2014). Therapeutic delivery of miR-200c enhances radiosensitivity in lung cancer. Molecular Therapy, 22(8), 1494–1503.
Moitra, K. (2015). Overcoming multidrug resistance in cancer stem cells. BioMed Research International, 2015.
Abdullah, L. N., & Chow, E. K. H. (2013). Mechanisms of chemoresistance in cancer stem cells. Clinical and Translational Medicine, 2, 3.
Pattabiraman, D. R., Bierie, B., Kober, K. I., Thiru, P., Krall, J. A., Zill, C., et al. (2016). Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science, 351(6277) aad3680.
Jordan, N. V., Bardia, A., Wittner, B. S., Benes, C., Ligorio, M., Zheng, Y., et al. (2016). HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature, 537(7618), 102–106.
Chaffer, C. L., Brueckmann, I., Scheel, C., Kaestli, A. J., Wiggins, P. A., Rodrigues, L. O., et al. (2011). Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proceedings of the National Academy of Sciences of the United States of America, 108(19), 7950–7955.
Funahashi, Y., Okamoto, K., Adachi, Y., Semba, T., Uesugi, M., Ozawa, Y., et al. (2014). Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Science, 105(10), 1334–1342.
Yoshida, T., Ozawa, Y., Kimura, T., Sato, Y., Kuznetsov, G., Xu, S., et al. (2014). Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. British Journal of Cancer, 110(6), 1497–1505.
Wang, C., Jiang, K., Kang, X., Gao, D., Sun, C., Li, Y., et al. (2012). Tumor-derived secretory clusterin induces epithelial-mesenchymal transition and facilitates hepatocellular carcinoma metastasis. The International Journal of Biochemistry & Cell Biology, 44(12), 2308–2320.
Tremblay, G., Malouin, M., Grothe, S., Kalbakji, A., Roy, S., Pagé, M., et al. (2010). Abstract 1467: AB-16B5, a therapeutic monoclonal antibody against human clusterin that blocks the epithelial-to-mesenchymal transition. Cancer Research, 70(8 Supplement), 1467 [10.1158/1538-7445.AM10-1467].
Tremblay, G. B., Viau, E., & Filion, M. (2012). Abstract LB-297: the EMT inhibitor AB-16B5 interacts with specific isoforms of secreted clusterin. Cancer Research, 72(8 Supplement) LB-297. [10.1158/1538-7445.AM2012-LB-297].
Jimeno, A., Gordon, M. S., Chugh, R., Messersmith, W. A., Mendelson, D. S., Dupont, J., et al. (2014). Abstract 2505; a first-in-human phase 1 study of anticancer stem cell agent OMP-54F28 (FZD8-Fc), decoy receptor for WNT ligands, in patients with advanced solid tumors. Journal of Clinical Oncology, 32, 5s.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chaffer, C.L., San Juan, B.P., Lim, E. et al. EMT, cell plasticity and metastasis. Cancer Metastasis Rev 35, 645–654 (2016). https://doi.org/10.1007/s10555-016-9648-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-016-9648-7