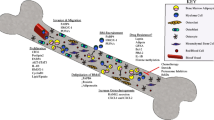
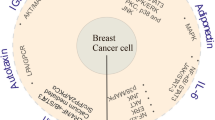
Abstract
Adipocytes are important but underappreciated components of bone marrow microenvironment, and their numbers greatly increase with age, obesity, and associated metabolic pathologies. Age and obesity are also significant risk factors for development of metastatic prostate cancer. Adipocytes are metabolically active cells that secrete adipokines, growth factors, and inflammatory mediators; influence behavior and function of neighboring cells; and have a potential to disturb local milleu and dysregulate normal bone homeostasis. Increased marrow adiposity has been linked to bone marrow inflammation and osteoporosis of the bone, but its effects on growth and progression of prostate tumors that have metastasized to the skeleton are currently not known. This review focuses on fat-bone relationship in a context of normal bone homeostasis and metastatic tumor growth in bone. We discuss effects of marrow fat cells on bone metabolism, hematopoiesis, and inflammation. Special attention is given to CCL2- and COX-2-driven pathways and their potential as therapeutic targets for bone metastatic disease.



Similar content being viewed by others
References
Lecka-Czernik, B. (2011). Marrow fat metabolism is linked to the systemic energy metabolism. Bone, 50(2), 534–539.
Lee, N. K., Sowa, H., Hinoi, E., Ferron, M., Ahn, J. D., Confavreux, C., Dacquin, R., Mee, P. J., McKee, M. D., Jung, D. Y., Zhang, Z., Kim, J. K., Mauvais-Jarvis, F., Ducy, P., & Karsenty, G. (2007). Endocrine regulation of energy metabolism by the skeleton. Cell, 130, 456–469.
Weilbaecher, K. N., Guise, T. A., & McCauley, L. K. (2011). Cancer to bone: a fatal attraction. Nature Reviews, 11, 411–425.
Roodman, G. D. (2004). Mechanisms of bone metastasis. New England Journal of Medicine, 350, 1655–1664.
Coleman, R. E. (2001). Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treatment Reviews, 27, 165–176.
Tuohimaa, P., Tenkanen, L., Syvala, H., Lumme, S., Hakulinen, T., Dillner, J., & Hakama, M. (2007). Interaction of factors related to the metabolic syndrome and vitamin D on risk of prostate cancer. Cancer Epidemiology, Biomarkers and Prevention, 16, 302–307.
Amling, C. L. (2005). Relationship between obesity and prostate cancer. Current Opinion in Urology, 15, 167–171.
Gong, Z., Neuhouser, M. L., Goodman, P. J., Albanes, D., Chi, C., Hsing, A. W., Lippman, S. M., Platz, E. A., Pollak, M. N., Thompson, I. M., & Kristal, A. R. (2006). Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiology, Biomarkers and Prevention, 15, 1977–1983.
Hsing, A. W., Chua, S., Jr., Gao, Y. T., Gentzschein, E., Chang, L., Deng, J., & Stanczyk, F. Z. (2001). Prostate cancer risk and serum levels of insulin and leptin: a population-based study. Journal of the National Cancer Institute, 93, 783–789.
Hsing, A. W., Sakoda, L. C., & Chua, S., Jr. (2007). Obesity, metabolic syndrome, and prostate cancer. The American Journal of Clinical Nutrition., 86, s843–857.
Hubbard, J. S., Rohrmann, S., Landis, P. K., Metter, E. J., Muller, D. C., Andres, R., Carter, H. B., & Platz, E. A. (2004). Association of prostate cancer risk with insulin, glucose, and anthropometry in the Baltimore longitudinal study of aging. Urology, 63, 253–258.
Kurahashi, N., Iwasaki, M., Sasazuki, S., Otani, T., Inoue, M., & Tsugane, S. (2006). Association of body mass index and height with risk of prostate cancer among middle-aged Japanese men. British Journal of Cancer, 94, 740–742.
Rodriguez, C., Freedland, S. J., Deka, A., Jacobs, E. J., McCullough, M. L., Patel, A. V., Thun, M. J., & Calle, E. E. (2007). Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiology, Biomarkers and Prevention, 16, 63–69.
Scosyrev, E., Messing, E. M., Mohile, S., Golijanin, D., & Wu, G. (2012). Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer, 118, 3062–3070.
Scosyrev, E., Wu, G., Mohile, S., & Messing, E. M. (2012). Prostate-specific antigen screening for prostate cancer and the risk of overt metastatic disease at presentation : Analysis of trends over time. Cancer, 118, 5768–5776.
Putnam, S. D., Cerhan, J. R., Parker, A. S., Bianchi, G. D., Wallace, R. B., Cantor, K. P., & Lynch, C. F. (2000). Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Annals of Epidemiology, 10, 361–369.
Dal Maso, L., Zucchetto, A., La Vecchia, C., Montella, M., Conti, E., Canzonieri, V., Talamini, R., Tavani, A., Negri, E., Garbeglio, A., & Franceschi, S. (2004). Prostate cancer and body size at different ages: an Italian multicentre case–control study. British Journal of Cancer, 90, 2176–2180.
MacInnis, R. J., English, D. R., Gertig, D. M., Hopper, J. L., & Giles, G. G. (2003). Body size and composition and prostate cancer risk. Cancer Epidemiology, Biomarkers and Prevention, 12, 1417–1421.
Freedland, S. J., Banez, L. L., Sun, L. L., Fitzsimons, N. J., & Moul, J. W. (2009). Obese men have higher-grade and larger tumors: an analysis of the duke prostate center database. Prostate Cancer and Prostatic Diseases, 12, 259–263.
Amling, C. L., Riffenburgh, R. H., Sun, L., Moul, J. W., Lance, R. S., Kusuda, L., Sexton, W. J., Soderdahl, D. W., Donahue, T. F., Foley, J. P., Chung, A. K., & McLeod, D. G. (2004). Pathologic variables and recurrence ates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. Journal of Clinical Oncology, 22, 439–445.
Bassett, W. W., Cooperberg, M. R., Sadetsky, N., Silva, S., DuChane, J., Pasta, D. J., Chan, J. M., Anast, J. W., Carroll, P. R., & Kane, C. J. (2005). Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology, 66, 1060–1065.
Freedland, S. J., Aronson, W. J., Kane, C. J., Presti, J. C., Jr., Amling, C. L., Elashoff, D., & Terris, M. K. (2004). Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. Journal of Clinical Oncology, 22, 446–453.
Freedland, S. J., Grubb, K. A., Yiu, S. K., Humphreys, E. B., Nielsen, M. E., Mangold, L. A., Isaacs, W. B., & Partin, A. W. (2005). Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. The Journal of Urology, 174, 919–922.
Palma, D., Pickles, T., & Tyldesley, S. (2007). Obesity as a predictor of biochemical recurrence and survival after radiation therapy for prostate cancer. BJU International, 100, 315–319.
Stroup, S. P., Cullen, J., Auge, B. K., L’Esperance, J. O., & Kang, S. K. (2007). Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer, 110, 1003–1009.
Rodriguez, C., Patel, A. V., Calle, E. E., Jacobs, E. J., Chao, A., & Thun, M. J. (2001). Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiology, Biomarkers and Prevention, 10, 345–353.
Calle, E. E., Rodriguez, C., Walker-Thurmond, K., & Thun, M. J. (2003). Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine, 348, 1625–1638.
Gong, Z., Agalliu, I., Lin, D. W., Stanford, J. L., & Kristal, A. R. (2007). Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer, 109, 1192–1202.
Keto, C. J., Aronson, W. J., Terris, M. K., Presti, J. C., Kane, C. J., Amling, C. L., & Freedland, S. J. (2012). Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU International, 110, 492–498.
Crujeiras, A. B., Diaz-Lagares, A., Carreira, M. C., Amil, M., & Casanueva, F. F. (2013). Oxidative stress associated to dysfunctional adipose tissue: a potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radical Research, 47, 243–256.
Gueron, G., De Siervi, A., & Vazquez, E. (2012). Advanced prostate cancer: reinforcing the strings between inflammation and the metastatic behavior. Prostate Cancer and Prostatic Diseases, 15, 213–221.
Nelson, W. G., DeWeese, T. L., & DeMarzo, A. M. (2002). The diet, prostate inflammation, and the development of prostate cancer. Cancer Metastasis Reviews, 21, 3–16.
Imbriaco, M., Larson, S. M., Yeung, H. W., Mawlawi, O. R., Erdi, Y., Venkatraman, E. S., & Scher, H. I. (1998). A new parameter for measuring metastatic bone involvement by prostate cancer: the bone scan index. Clinical Cancer Research, 4, 1765–1772.
Schneider, A., Kalikin, L. M., Mattos, A. C., Keller, E. T., Allen, M. J., Pienta, K. J., & McCauley, L. K. (2005). Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology, 146, 1727–1736.
Guise, T. A., Mohammad, K. S., Clines, G., Stebbins, E. G., Wong, D. H., Higgins, L. S., Vessella, R., Corey, E., Padalecki, S., Suva, L., & Chirgwin, J. M. (2006). Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clinical Cancer Research, 12, 6213s–6216s.
Park, S. I., Liao, J., Berry, J. E., Li, X., Koh, A. J., Michalski, M. E., Eber, M. R., Soki, F. N., Sadler, D., Sud, S., Tisdelle, S., Daignault, S. D., Nemeth, J. A., Snyder, L. A., Wronski, T. J., Pienta, K. J., & McCauley, L. K. (2012). Cyclophosphamide creates a receptive microenvironment for prostate cancer skeletal metastasis. Cancer Research, 72, 2522–2532.
Brown, M. D., Hart, C. A., Gazi, E., Bagley, S., & Clarke, N. W. (2006). Promotion of prostatic metastatic migration towards human bone marrow stoma by omega 6 and its inhibition by omega 3 PUFAS. British Journal of Cancer, 94, 842–853.
Gazi, E., Gardner, P., Lockyer, N. P., Hart, C. A., Brown, M. D., & Clarke, N. W. (2007). Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. Journal of Lipid Research, 48, 1846–1856.
Gimble, J. M., & Nuttall, M. E. (2004). Bone and fat: old questions, new insights. Endocrine, 23, 183–188.
Rosen, C., & Bouxsein, M. (2006). Mechanisms of disease: is osteoporosis the obesity of bone? Nature Clinical Practice Rheumatology, 2, 35–43.
Beresford, J. N., Bennett, J. H., Devlin, C., Leboy, P. S., & Owen, M. E. (1992). Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. Journal of Cell Science, 102(Pt 2), 341–351.
Owen, M. (1988). Marrow stromal stem cells. Journal of Cell Science, 10, 63–76 (Supplement).
Kawai, M., de Paula, F., & Rosen, C. (2012). New insights into osteoporosis: the bone-fat connection. Journal of Internal Medicine, 272, 317–329.
Lecka-Czernik, B., Rosen, C. J., & Kawai, M. (2010). Skeletal aging and the adipocyte program: new insights from an “old” molecule. Cell Cycle, 9, 3648–3654.
Rosen, C. J., Ackert-Bicknell, C., Rodriguez, J. P., & Pino, A. M. (2009). Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Critical Reviews in Eukaryotic Gene Expression, 19, 109–124.
Jilka, R. L. (2002). Osteoblast progenitor fate and age-related bone loss. Journal of Musculoskeletal & Neuronal Interactions, 2, 581–583.
Oh, S., Sul, O., Kim, Y., Kim, H., Yu, R., Suh, J., & Choi, H. (2010). Saturated fatty acids enhance osteoclast survival. Journal of Lipid Research, 51, 892–899.
Chen, J., Lazarenko, O., Wu, X., Tong, Y., Blackburn, M., Shankar, K., Badger, T., & Ronis, M. (2010). Obesity reduces bone density associated with activation of PPARγ and suppression of Wnt/β-catenin in rapidly growing male rats. PLoS ONE, 5, e13704.
Moerman, E. J., Teng, K., Lipschitz, D. A., & Lecka-Czernik, B. (2004). Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell, 3, 379–389.
Duque, G. (2007). As a matter of fat: new perspectives on the understanding of age-related bone loss. BoneKEy-Osteovision., 4, 129–140.
Wan, Y., Chong, L. W., & Evans, R. M. (2007). PPAR-gamma regulates osteoclastogenesis in mice. Nature Medicine, 13, 1496–1503.
Lecka-Czernik, B. (2010). PPARs in bone: the role in bone cell differentiation and regulation of energy metabolism. Current Osteoporosis Reports, 8, 84–90.
Cao, J. (2011). Effects of obesity on bone metabolism. Journal of Orthopaedic Surgery and Research, 6, 30. doi:10.1186/1749-799X-6-30.
Villareal, D. T., Apovian, C. M., Kushner, R. F., Klein, S., American Society for Nutrition, & Naaso TOS. (2005). Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obesity Research, 13, 1849–1863.
Bonewald, L. F., & Johnson, M. L. (2008). Osteocytes, mechanosensing and Wnt signaling. Bone, 42, 606–615.
Nielson, C. M., Srikanth, P., & Orwoll, E. S. (2012). Obesity and fracture in men and women: an epidemiologic perspective. Journal of Bone and Mineral Research, 27, 1–10.
Hsu, Y. H., Venners, S. A., Terwedow, H. A., Feng, Y., Niu, T., Li, Z., Laird, N., Brain, J. D., Cummings, S. R., Bouxsein, M. L., Rosen, C. J., & Xu, X. (2006). Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. The American Journal of Clinical Nutrition, 83, 146–154.
Owusu, W., Willett, W., Ascherio, A., Spiegelman, D., Rimm, E., Feskanich, D., & Colditz, G. (1998). Body anthropometry and the risk of hip and wrist fractures in men: results from a prospective study. Obesity Research, 6, 12–19.
Kugel, H., Jung, C., Schulte, O., & Heindel, W. (2001). Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. Journal of Magnetic Resonance Imaging, 13, 263–268.
Justesen, J., Stenderup, K., Ebbesen, E. N., Mosekilde, L., Steiniche, T., & Kassem, M. (2001). Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology, 2, 165–171.
Cao, J., Sun, L., & Gao, H. (2010). Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Annals of the New York Academy of Sciences, 1192, 292–297.
Cao, J. J., Gregoire, B. R., & Gao, H. (2009). High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone, 44, 1097–1104.
Halade, G., Rahman, M., Williams, P., & Fernandes, G. (2010). High fat diet-induced animal model of age-associated obesity and osteoporosis. Journal of Nutritional Biochemistry, 21, 1162–1169.
Halade, G., El Jamali, A., Williams, P., Fajardo, R., & Fernandes, G. (2011). Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Experimental Gerontology, 46, 43–52.
Kyung, T., Lee, J., Phan, T., Yu, R., & Choi, H. (2009). Osteoclastogenesis by bone marrow-derived macrophages is enhanced in obese mice. Journal of Nutrition, 139, 502–506.
Krings, A., Rahman, S., Huang, S., Lu, Y., Czernik, P. J., & Lecka-Czernik, B. (2012). Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone, 50, 546–552.
Fazeli, P. K., Horowitz, M. C., MacDougald, O. A., Scheller, E. L., Rodeheffer, M. S., Rosen, C. J., & Klibanski, A. (2013). Marrow fat and bone–new perspectives. The Journal of Clinical Endocrinology and Metabolism, 98, 935–945.
Gimble, J. M., Zvonic, S., Floyd, Z. E., Kassem, M., & Nuttall, M. E. (2006). Playing with bone and fat. Journal of Cellular Biochemistry, 98, 251–266.
Lecka-Czernik, B., Gubrij, I., Moerman, E. J., Kajkenova, O., Lipschitz, D. A., Manolagas, S. C., & Jilka, R. L. (1999). Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. Journal of Cellular Biochemistry, 74, 357–371.
Shockley, K. R., Lazarenko, O. P., Czernik, P. J., Rosen, C. J., Churchill, G. A., & Lecka-Czernik, B. (2009). PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. Journal of Cellular Biochemistry, 106, 232–246.
Gimble, J. M. (1990). The function of adipocytes in the bone marrow stroma. The New Biologist, 2, 304–312.
Paula, F. J., & Rosen, C. J. (2010). Obesity, diabetes mellitus and last but not least, osteoporosis. Arquivos Brasileiros de Endocrinologia e Metabologia, 54, 150–157.
Roodman, G. D. (2012). Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Review, 31(3–4), 569–78.
Trottier, M. D., Naaz, A., Li, Y., & Fraker, P. J. (2012). Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proceedings of the National Academy of Sciences of the United States of America, 109, 7622–7629.
van Dielen, F. M., van’t Veer, C., Schols, A. M., Soeters, P. B., Buurman, W. A., & Greve, J. W. (2001). Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. International Journal of Obesity and Related Metabolic Disorders, 25, 1759–1766.
Barbour, K. E., Zmuda, J. M., Boudreau, R., Strotmeyer, E. S., Horwitz, M. J., Evans, R. W., Kanaya, A. M., Harris, T. B., Bauer, D. C., & Cauley, J. A. (2011). Adipokines and the risk of fracture in older adults. Journal of Bone and Mineral Research, 26, 1568–1576.
Motyl, K. J., & Rosen, C. J. (2012). Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie, 94, 2089–2096.
Sadie-Van Gijsen, H., Crowther, N. J., Hough, F. S., & Ferris, W. F. (2012). The interrelationship between bone and fat: from cellular see-saw to endocrine reciprocity. Cellular and Molecular Life Sciences. doi:10.1007/s00018-012-1211-2.
Gordeladze, J. O., Drevon, C. A., Syversen, U., & Reseland, J. E. (2002). Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. Journal of Cellular Biochemistry, 85, 825–836.
Scheller, E. L., Song, J., Dishowitz, M. I., Soki, F. N., Hankenson, K. D., & Krebsbach, P. H. (2010). Leptin functions peripherally to regulate differentiation of mesenchymal progenitor cells. Stem Cells (Dayton, Ohio), 28, 1071–1080.
Thomas, T., Gori, F., Khosla, S., Jensen, M. D., Burguera, B., & Riggs, B. L. (1999). Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology, 140, 1630–1638.
Hamrick, M. W. (2004). Leptin, bone mass, and the thrifty phenotype. Journal of Bone and Mineral Research, 19, 1607–1611.
Hamrick, M. W., Della-Fera, M. A., Choi, Y. H., Pennington, C., Hartzell, D., & Baile, C. A. (2005). Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. Journal of Bone and Mineral Research, 20, 994–1001.
Berg, A. H., Combs, T. P., & Scherer, P. E. (2002). ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends in Endocrinology and Metabolism, 13, 84–89.
Reid, I. R. (2010). Fat and bone. Archives of Biochemistry and Biophysics, 503, 20–27.
Shinoda, Y., Yamaguchi, M., Ogata, N., Akune, T., Kubota, N., Yamauchi, T., Terauchi, Y., Kadowaki, T., Takeuchi, Y., Fukumoto, S., Ikeda, T., Hoshi, K., Chung, U., Nakamura, K., & Kawaguchi, H. (2006). Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. Journal of Cellular Biochemistry, 99, 196–208.
Wolf, A. M., Wolf, D., Rumpold, H., Enrich, B., & Tilg, H. (2004). Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochemical and Biophysical Research Communications, 323, 630–635.
Kanazawa, I. (2012). Adiponectin in metabolic bone disease. Current Medicinal Chemistry, 19, 5481–5492.
Oshima, K., Nampei, A., Matsuda, M., Iwaki, M., Fukuhara, A., Hashimoto, J., Yoshikawa, H., & Shimomura, I. (2005). Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochemical and Biophysical Research Communications, 331, 520–526.
Williams, G. A., Wang, Y., Callon, K. E., Watson, M., Lin, J. M., Lam, J. B., Costa, J. L., Orpe, A., Broom, N., Naot, D., Reid, I. R., & Cornish, J. (2009). In vitro and in vivo effects of adiponectin on bone. Endocrinology, 150, 3603–3610.
de Heredia, F. P., Gomez-Martinez, S., & Marcos, A. (2012). Obesity, inflammation and the immune system. The Proceedings of the Nutrition Society, 71, 332–338.
Harte, A. L., Tripathi, G., Piya, M. K., Barber, T. M., Clapham, J. C., Al-Daghri, N., Al-Disi, D., Kumsaiyai, W., Saravanan, P., Fowler, A. E., Oh, J. P., Kumar, S., & McTernan, P. G. (2013). NFkappaB as a potent regulator of inflammation in human adipose tissue, influenced by depot, adiposity, T2DM status, and TNFalpha. Obesity. doi:10.1002/oby.20336 (Silver Spring, MD).
Mathis, D. (2013). Immunological goings-on in visceral adipose tissue. Cell Metabolism, 17, 851–859.
Corre, J., Planat-Benard, V., Corberand, J. X., Penicaud, L., Casteilla, L., & Laharrague, P. (2004). Human bone marrow adipocytes support complete myeloid and lymphoid differentiation from human CD34 cells. British Journal of Haematology, 127, 344–347.
Gimble, J. M., & Nuttall, M. E. (2012). The relationship between adipose tissue and bone metabolism. Clinical Biochemistry, 45, 874–879.
Naveiras, O., Nardi, V., Wenzel, P. L., Hauschka, P. V., Fahey, F., & Daley, G. Q. (2009). Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature, 460, 259–263.
Omatsu, Y., Sugiyama, T., Kohara, H., Kondoh, G., Fujii, N., Kohno, K., & Nagasawa, T. (2010). The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity, 33, 387–399.
Belaid-Choucair, Z., Lepelletier, Y., Poncin, G., Thiry, A., Humblet, C., Maachi, M., Beaulieu, A., Schneider, E., Briquet, A., Mineur, P., Lambert, C., Mendes-Da-Cruz, D., Ahui, M. L., Asnafi, V., Dy, M., Boniver, J., Nusgens, B. V., Hermine, O., & Defresne, M. P. (2008). Human bone marrow adipocytes block granulopoiesis through neuropilin-1-induced granulocyte colony-stimulating factor inhibition. Stem Cells (Dayton, Ohio), 26, 1556–1564.
Miharada, K., Hiroyama, T., Sudo, K., Danjo, I., Nagasawa, T., & Nakamura, Y. (2008). Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. Journal of Cellular Physiology, 215, 526–537.
DiMascio, L., Voermans, C., Uqoezwa, M., Duncan, A., Lu, D., Wu, J., Sankar, U., & Reya, T. (2007). Identification of adiponectin as a novel hemopoietic stem cell growth factor. The Journal of Immunology, 178, 3511–3520.
Hotamisligil, G. S., Shargill, N. S., & Spiegelman, B. M. (1993). Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science, 259, 87–91.
Pang, W. W., Price, E. A., Sahoo, D., Beerman, I., Maloney, W. J., Rossi, D. J., Schrier, S. L., & Weissman, I. L. (2011). Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proceedings of the National Academy of Sciences of the United States of America, 108, 20012–20017.
Ogawa, T., Kitagawa, M., & Hirokawa, K. (2000). Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mechanisms of Ageing and Development, 117, 57–68.
Serafini, P., De Santo, C., Marigo, I., Cingarlini, S., Dolcetti, L., Gallina, G., Zanovello, P., & Bronte, V. (2004). Derangement of immune responses by myeloid suppressor cells. Cancer Immunology, Immunotherapy, 53, 64–72.
Roca, H., Varsos, Z. S., Sud, S., Craig, M. J., Ying, C., & Pienta, K. J. (2009). CCL2 and interleukin-6 promote survival of human CD11b + peripheral blood mononuclear cells and induce M2-type macrophage polarization. Journal of Biological Chemistry, 284, 34342–34354.
Johnson, A. R., Milner, J. J., & Makowski, L. (2012). The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunological Reviews, 249, 218–238.
Stienstra, R., Tack, C. J. K. T., Joosten, L. A., & Netea, M. G. (2012). The inflammasome puts obesity in the danger zone. Cell Metabolism, 15, 10–18.
Tchernof, A., & Despres, J. P. (2013). Pathophysiology of human visceral obesity: an update. Physiological Reviews, 93, 359–404.
Khosla, S. (2001). Minireview: the OPG/RANKL/RANK system. Endocrinology, 142, 5050–5055.
Kimble, R. B., Matayoshi, A. B., Vannice, J. L., Kung, V. T., Williams, C., & Pacifici, R. (1995). Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology, 136, 3054–3061.
Axmann, R. (2009). Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis and Rheumatism, 60, 2747–2756. doi:10.1002/art.24781.
Cauley, J., Danielson, M., Boudreau, R., Forrest, K., Zmuda, J., Pahor, M., Tylavsky, F., Cummings, S., Harris, T., Newman, A., & Health ABC Study. (2007). Inflammatory markers and incident fracture risk in older Men and women: the health aging and body composition study. Journal of Bone and Mineral Research, 22, 1088–1095.
Neels, J. G., & Olefsky, J. M. (2006). Inflamed fat: what starts the fire? Journal of Clinical Investigation, 116, 33–35.
Weisberg, S. P., McCann, D., Desai, M., Rosenbaum, M., Leibel, R. L., & Ferrante, A. W., Jr. (2003). Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation, 112, 1796–1808.
Craig, M. J., & Loberg, R. D. (2006). CCL2 (monocyte chemoattractant protein-1) in cancer bone metastases. Cancer Metastasis Reviews, 25, 611–619.
Weisberg, S. P., Hunter, D., Huber, R., Lemieux, J., Slaymaker, S., Vaddi, K., Charo, I., Leibel, R. L., & Ferrante, A. W., Jr. (2006). CCR2 modulates inflammatory and metabolic effects of high-fat feeding. Journal of Clinical Investigation, 116, 115–124.
Kamei, N., Tobe, K., Suzuki, R., Ohsugi, M., Watanabe, T., Kubota, N., Ohtsuka-Kowatari, N., Kumagai, K., Sakamoto, K., Kobayashi, M., Yamauchi, T., Ueki, K., Oishi, Y., Nishimura, S., Manabe, I., Hashimoto, H., Ohnishi, Y., Ogata, H., Tokuyama, K., Tsunoda, M., Ide, T., Murakami, K., Nagai, R., & Kadowaki, T. (2006). Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. Journal of Biological Chemistry, 281, 26602–26614.
Kanda, H., Tateya, S., Tamori, Y., Kotani, K., Hiasa, K., Kitazawa, R., Kitazawa, S., Miyachi, H., Maeda, S., Egashira, K., & Kasuga, M. (2006). MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. Journal of Clinical Investigation, 116, 1494–1505.
Hopwood, B., Tsykin, A., Findlay, D. M., & Fazzalari, N. L. (2009). Gene expression profile of the bone microenvironment in human fragility fracture bone. Bone, 44, 87–101.
Kim, M. S., Day, C. J., & Morrison, N. A. (2005). MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. Journal of Biological Chemistry, 280, 16163–16169.
Graves, D. T., Jiang, Y., & Valente, A. J. (1999). The expression of monocyte chemoattractant protein-1 and other chemokines by osteoblasts. Frontiers in Bioscience, 4, D571–580.
Posner, L. J., Miligkos, T., Gilles, J. A., Carnes, D. L., Taddeo, D. R., & Graves, D. T. (1997). Monocyte chemoattractant protein-1 induces monocyte recruitment that is associated with an increase in numbers of osteoblasts. Bone, 21, 321–327.
Sul, O. J., Ke, K., Kim, W. K., Kim, S. H., Lee, S. C., Kim, H. J., Kim, S. Y., Suh, J. H., & Choi, H. S. (2012). Absence of MCP-1 leads to elevated bone mass via impaired actin ring formation. Journal of Cellular Physiology, 227, 1619–1627.
Blackwell, K. A., Raisz, L. G., & Pilbeam, C. C. (2010). Prostaglandins in bone: bad cop, good cop? Trends in Endocrinology and Metabolism, 21, 294–301.
Wang, D., & Dubois, R. N. (2010). Eicosanoids and cancer. Nature Reviews., 10, 181–193.
Naik, A. A., Xie, C., Zuscik, M. J., Kingsley, P., Schwarz, E. M., Awad, H., Guldberg, R., Drissi, H., Puzas, J. E., Boyce, B., Zhang, X., & O’Keefe, R. J. (2009). Reduced COX-2 expression in aged mice is associated with impaired fracture healing. Journal of Bone and Mineral Research, 24, 251–264.
Simon, A. M., Manigrasso, M. B., & O’Connor, J. P. (2002). Cyclo-oxygenase 2 function is essential for bone fracture healing. Journal of Bone and Mineral Research, 17, 963–976.
Tian, X. Y., Zhang, Q., Zhao, R., Setterberg, R. B., Zeng, Q. Q., Iturria, S. J., Ma, Y. F., & Jee, W. S. (2008). Continuous PGE2 leads to net bone loss while intermittent PGE2 leads to net bone gain in lumbar vertebral bodies of adult female rats. Bone, 42, 914–920.
Chen, Y. C., Sosnoski, D. M., Gandhi, U. H., Novinger, L. J., Prabhu, K. S., & Mastro, A. M. (2009). Selenium modifies the osteoblast inflammatory stress response to bone metastatic breast cancer. Carcinogenesis, 30, 1941–1948.
Hsieh, P. S., Lu, K. C., Chiang, C. F., & Chen, C. H. (2010). Suppressive effect of COX2 inhibitor on the progression of adipose inflammation in high-fat-induced obese rats. European Journal of Clinical Investigation, 40, 164–171.
Anderson, G. D., Hauser, S. D., McGarity, K. L., Bremer, M. E., Isakson, P. C., & Gregory, S. A. (1996). Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. Journal of Clinical Investigation, 97, 2672–2679.
Sinha, P., Clements, V. K., Fulton, A. M., & Ostrand-Rosenberg, S. (2007). Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Research, 67, 4507–4513.
Muta, M., Matsumoto, G., Nakashima, E., & Toi, M. (2006). Mechanical analysis of tumor growth regression by the cyclooxygenase-2 inhibitor, DFU, in a Walker256 rat tumor model: importance of monocyte chemoattractant protein-1 modulation. Clinical Cancer Research, 12, 264–272.
Fujita, M., Kohanbash, G., Fellows-Mayle, W., Hamilton, R. L., Komohara, Y., Decker, S. A., Ohlfest, J. R., & Okada, H. (2011). COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Research, 71, 2664–2674.
Eliopoulos, A. G., Dumitru, C. D., Wang, C. C., Cho, J., & Tsichlis, P. N. (2002). Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO Journal, 21, 4831–4840.
Nakao, S., Kuwano, T., Tsutsumi-Miyahara, C., Ueda, S., Kimura, Y. N., Hamano, S., Sonoda, K. H., Saijo, Y., Nukiwa, T., Strieter, R. M., Ishibashi, T., Kuwano, M., & Ono, M. (2005). Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. Journal of Clinical Investigation, 115, 2979–2991.
Tajima, T., Murata, T., Aritake, K., Urade, Y., Hirai, H., Nakamura, M., Ozaki, H., & Hori, M. (2008). Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2). The Journal of Pharmacology and Experimental Therapeutics, 326, 493–501.
Tanaka, S., Tatsuguchi, A., Futagami, S., Gudis, K., Wada, K., Seo, T., Mitsui, K., Yonezawa, M., Nagata, K., Fujimori, S., Tsukui, T., Kishida, T., & Sakamoto, C. (2006). Monocyte chemoattractant protein 1 and macrophage cyclooxygenase 2 expression in colonic adenoma. Gut, 55, 54–61.
Fruhbeck, G., Gomez-Ambrosi, J., Muruzabal, F. J., & Burrell, M. A. (2001). The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. American Journal of Physiology., 280, E827–847.
Kaneko, A., Satoh, Y., Tokuda, Y., Fujiyama, C., Udo, K., & Uozumi, J. (2010). Effects of adipocytes on the proliferation and differentiation of prostate cancer cells in a 3-D culture model. International Journal of Urology, 17, 369–376.
Nieman, K., Kenny, H., Penicka, C., Ladanyi, A., Buell-Gutbrod, R., Zillhardt, M., Romero, I., Carey, M., Mills, G., Hotamisligil, G., Yamada, S., Peter, M., Gwin, K., & Lengyel, E. (2011). Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature Medicine., 17, 1498–1503.
Tokuda, Y., Satoh, Y., Fujiyama, C., Toda, S., Sugihara, H., & Masaki, Z. (2003). Prostate cancer cell growth is modulated by adipocyte-cancer cell interaction. BJU International, 91, 716–720.
Dirat, B., Bochet, L., Dabek, M., Daviaud, D., Dauvillier, S., Majed, B., Wang, Y., Meulle, A., Salles, B., Le Gonidec, S., Garrido, I., Escourrou, G., Valet, P., & Muller, C. (2011). Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Research, 71, 2455–2465.
Nieman, K. M., Romero, I. L., Van Houten, B., & Lengyel, E. (2013). Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica et Biophysica Acta, 1831, 1533–41.
Khandekar, M. J., Cohen, P., & Spiegelman, B. M. (2011). Molecular mechanisms of cancer development in obesity. Nature Reviews., 11, 886–895.
Zadra, G., Photopoulos, C., & Loda, M. (2013). The fat side of prostate cancer. Biochimica et Biophysica Acta, 1831, 1518–1532.
Llorente, A., Skotland, T., Sylvänne, T., Kauhanen, D., Rog, T., Orłowski, A., Vattulainen, I., Ekroos, K., & Sandvig, K. (2013). Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochimica et Biophysica Acta, 1831, 1302–9.
Buzzai, M., Bauer, D. E., Jones, R. G., Deberardinis, R. J., Hatzivassiliou, G., Elstrom, R. L., & Thompson, C. B. (2005). The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene, 24, 4165–4173.
Suburu, J., & Chen, Y. Q. (2012). Lipids and prostate cancer. Prostaglandins & Other Lipid Mediators, 98, 1–10.
Liu, Y. (2006). Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer and Prostatic Diseases., 9, 230–234.
Herroon, M., Rajagurubandara, E., Hardaway, A., Powell, K., Turchick, A., Feldmann, D., & Podgorski, I. (2013). Bone marrow adipocytes promote tumor growth and survival in bone via FABP4-dependent mechanisms. Oncotarget, 4, 2108–2123.
Paz-Filho, G., Lim, E. L., Wong, M. L., & Licinio, J. (2011). Associations between adipokines and obesity-related cancer. Frontiers in Bioscience, 16, 1634–1650.
Freedland, S. J., & Platz, E. A. (2007). Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiologic Reviews., 29, 88–97.
Hoda, M. R., Theil, G., Mohammed, N., Fischer, K., & Fornara, P. (2012). The adipocyte-derived hormone leptin has proliferative actions on androgen-resistant prostate cancer cells linking obesity to advanced stages of prostate cancer. Journal of Oncology., 2012, 280386.
Onuma, M., Bub, J. D., Rummel, T. L., & Iwamoto, Y. (2003). Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. Journal of Biological Chemistry, 278, 42660–42667.
Bussard, K., Gay, C., & Mastro, A. (2008). The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Reviews, 27, 41–55.
Thobe, M. N., Clark, R. J., Bainer, R. O., Prasad, S. M., & Rinker-Schaeffer, C. W. (2011). From prostate to bone: key players in prostate cancer bone metastasis. Cancers (Basel)., 3, 478–493.
Fain, J. N., Kanu, A., Bahouth, S. W., Cowan, G. S., Jr., Hiler, M. L., & Leffler, C. W. (2002). Comparison of PGE2, prostacyclin and leptin release by human adipocytes versus explants of adipose tissue in primary culture. Prostaglandins, Leukotrienes, and Essential Fatty Acids., 67, 467–473.
Fain, J. N., Leffler, C. W., & Bahouth, S. W. (2000). Eicosanoids as endogenous regulators of leptin release and lipolysis by mouse adipose tissue in primary culture. Journal of Lipid Research., 41, 1689–1694.
Goktas, S., Yilmaz, M. I., Caglar, K., Sonmez, A., Kilic, S., & Bedir, S. (2005). Prostate cancer and adiponectin. Urology, 65, 1168–1172.
Kelesidis, I., Kelesidis, T., & Mantzoros, C. S. (2006). Adiponectin and cancer: a systematic review. British Journal of Cancer., 94, 1221–1225.
Bub, J. D., Miyazaki, T., & Iwamoto, Y. (2006). Adiponectin as a growth inhibitor in prostate cancer cells. Biochemical and Biophysical Research Communications, 340, 1158–1166.
Mistry, T., Digby, J. E., Chen, J., Desai, K. M., & Randeva, H. S. (2006). The regulation of adiponectin receptors in human prostate cancer cell lines. Biochemical and Biophysical Research Communications, 348, 832–838.
Mistry, T., Digby, J. E., Desai, K. M., & Randeva, H. S. (2007). Obesity and prostate cancer: a role for adipokines. European Urology., 52, 46–53.
Mistry, T., Digby, J. E., Desai, K. M., & Randeva, H. S. (2008). Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU International, 101, 1317–1322.
Yokota, T., Meka, C. S., Medina, K. L., Igarashi, H., Comp, P. C., Takahashi, M., Nishida, M., Oritani, K., Miyagawa, J., Funahashi, T., Tomiyama, Y., Matsuzawa, Y., & Kincade, P. W. (2002). Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins. Journal of Clinical Investigation, 109, 1303–1310.
Yokota, T., Meka, C. S., Kouro, T., Medina, K. L., Igarashi, H., Takahashi, M., Oritani, K., Funahashi, T., Tomiyama, Y., Matsuzawa, Y., & Kincade, P. W. (2003). Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. The Journal of Immunology, 171, 5091–5099.
Gasparini, G., Longo, R., Sarmiento, R., & Morabito, A. (2003). Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? The Lancet Oncology, 4, 605–615.
Kalinski, P. (2012). Regulation of immune responses by prostaglandin E2. The Journal of Immunology, 188, 21–28.
Karavitis, J., Hix, L., Shi, Y., Schultz, R., Khazaie, K., & Zhang, M. (2012). Regulation of COX2 expression in mouse mammary tumor cells controls bone metastasis and PGE2-induction of regulatory T cell migration. PLoS ONE, 7, e46342.
Lucci, A., Krishnamurthy, S., Singh, B., Bedrosian, I., Meric-Bernstam, F., Reuben, J., Broglio, K., Mosalpuria, K., Lodhi, A., Vincent, L., & Cristofanilli, M. (2009). Cyclooxygenase-2 expression in primary breast cancers predicts dissemination of cancer cells to the bone marrow. Breast Cancer Research and Treatment, 117, 61–68.
Safina, A., Sotomayor, P., Limoge, M., Morrison, C., & Bakin, A. (2011). TAK1-TAB2 signaling contributes to bone destruction by breast carcinoma cells. Molecular Cancer Research, 9, 1042–1053.
Singh, B., Berry, J., Shoher, A., Ayers, G., Wei, C., & Lucci, A. (2007). COX-2 involvement in breast cancer metastasis to bone. Oncogene, 26, 3789–3796.
Li, Z., Schem, C., Shi, Y., Medina, D., & Zhang, M. (2008). Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clinical and Experimental Metastasis, 25, 389–400.
Lee, E., Choi, E., Kim, S., Park, J., Kim, H., Ha, K., Kim, Y., Kim, S., Choe, M., Kim, J., & Han, J. (2007). Cyclooxygenase-2 promotes cell proliferation, migration and invasion in U2OS human osteosarcoma cells. Experimental and Molecular Medicine, 39, 469–476.
Takita, M., Inada, M., Maruyama, T., & Miyaura, C. (2007). Prostaglandin E receptor EP4 antagonist suppresses osteolysis due to bone metastasis of mouse malignant melanoma cells. FEBS Letters, 581, 565–571.
Valcarcel, M., Mendoza, L., Hernandez, J. J., Carrascal, T., Salado, C., Crende, O., & Vidal-Vanaclocha, F. (2011). Vascular endothelial growth factor regulates melanoma cell adhesion and growth in the bone marrow microenvironment via tumor cyclooxygenase-2. Journal of Translational Medicine, 9, 142.
Liu, X. H., Kirschenbaum, A., Yao, S., Lee, R., Holland, J. F., & Levine, A. C. (2000). Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. The Journal of Urology., 164, 820–825.
Vo, B., Morton, D. S. K., Millena, A., Leath, C., & Khan, S. (2013). TGF-β effects on prostate cancer cell migration and invasion are mediated by PGE2 through activation of PI3K/AKT/mTOR pathway. Endocrinology, 154, 1768–1779.
Hiraga, T., Myoui, A., Choi, M. E., Yoshikawa, H., & Yoneda, T. (2006). Stimulation of cyclooxygenase-2 expression by bone-derived transforming growth factor-beta enhances bone metastases in breast cancer. Cancer Research, 66, 2067–2073.
Takahashi, T., Uehara, H., Bando, Y., & Izumi, K. (2008). Soluble EP2 neutralizes prostaglandin E2-induced cell signaling and inhibits osteolytic tumor growth. Molecular Cancer Therapeutics, 7, 2807–2816.
Liu, Q., Russell, M. R., Shahriari, K., Jernigan, D. L., Lioni, M. I., Garcia, F. U., & Fatatis, A. (2013). Interleukin-1beta promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Research, 73, 3297–3305.
Le Bitoux, M. A., & Stamenkovic, I. (2008). Tumor-host interactions: the role of inflammation. Histochemistry and Cell Biology., 130, 1079–1090.
Li, H. J., Reinhardt, F., Herschman, H. R., & Weinberg, R. A. (2012). Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discovery., 2, 840–855.
Loberg, R., Day, L., Harwood, J., Ying, C., St John, L., Giles, R., Neeley, C., & Pienta, K. (2006). CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia, 8, 578–586.
Loberg, R. D., Ying, C., Craig, M., Yan, L., Snyder, L. A., & Pienta, K. J. (2007). CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia, 9, 556–562.
Lu, Y., Cai, Z., Galson, D. L., Xiao, G., Liu, Y., George, D. E., Melhem, M. F., Yao, Z., & Zhang, J. (2006). Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate, 66, 1311–1318.
Herroon, M., Rajagurubandara, E. D. R., Chalasani, A., Hardaway, A., & Podgorski, I. (2013). Macrophage cathepsin K promotes prostate tumor progression in bone. Oncogene, 32, 1580–1593.
Qian, B. Z., Li, J., Zhang, H., Kitamura, T., Zhang, J., Campion, L. R., Kaiser, E. A., Snyder, L. A., & Pollard, J. W. (2011). CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature, 475, 222–5.
Zhang, J., Patel, L., & Pienta, K. (2010). CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine and Growth Factor Reviews, 21, 41–48.
Lu, Y., Cai, Z., Xiao, G., Keller, E., Mizokami, A., Yao, Z., Roodman, G., & Zhang, J. (2007). Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Research, 67, 3646–3653.
Li, X., Loberg, R., Liao, J., Ying, C., Snyder, L., Pienta, K., & McCauley, L. (2009). A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Research, 69, 1685–1692.
Mizutani, K., Sud, S., McGregor, N., Martinovski, G., Rice, B., Craig, M., Varsos, Z., Roca, H., & Pienta, K. (2009). The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia, 11, 1235–1242.
Lu, Y., Chen, Q., Corey, E., Xie, W., Fan, J., Mizokami, A., & Zhang, J. (2009). Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clinical and Experimental Metastasis, 26, 161–169.
Nalla, A., Estes, N., Patel, J., & Rao, J. (2011). N-cadherin mediates angiogenesis by regulating monocyte chemoattractant protein-1 expression via PI3K/Akt signaling in prostate cancer cells. Experimental Cell Research, 317, 2512–2521.
Hsieh, P. S., Jin, J. S., Chiang, C. F., Chan, P. C., Chen, C. H., & Shih, K. C. (2009). COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity, 17, 1150–1157 (Silver Spring, MD).
Bossard, M. J., Tomaszek, T. A., Thompson, S. K., Amegadzie, B. Y., Hanning, C. R., Jones, C., Kurdyla, J. T., McNulty, D. E., Drake, F. H., Gowen, M., & Levy, M. A. (1996). Proteolytic activity of human osteoclast cathepsin K. Expression, purification, activation, and substrate identification. Journal of Biological Chemistry, 271, 12517–12524.
Popivanova, B. K., Kostadinova, F. I., Furuichi, K., Shamekh, M. M., Kondo, T., Wada, T., Egashira, K., & Mukaida, N. (2009). Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Research, 69, 7884–7892.
Huang, M., Narita, S., Numakura, K., Tsuruta, H., Saito, M., Inoue, T., Horikawa, Y., Tsuchiya, N., & Habuchi, T. (2012). A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate, 72, 1779–1788.
Pienta, K., Machiels, J., Schrijvers, D., Alekseev, B., Shkolnik, M., Crabb, S., Li, S., Seetharam, S., Puchalski, T., Takimoto, C., Elsayed, Y., Dawkins, F., & de Bono, J. (2013). Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Investigational New Drugs, 32, 760–768.
Abedinpour, P., Baron, V. J. W., & Borgström, P. (2011). Regression of prostate tumors upon combination of hormone ablation therapy and celecoxib in vivo. Prostate, 71, 813–823.
Antonarakis, E., Heath, E., Walczak, J., Nelson, W., Fedor, H., De Marzo, A., Zahurak, M., Piantadosi, S., Dannenberg, A., Gurganus, R., Baker, S., Parnes, H., DeWeese, T., Partin, A., & Carducci, M. (2009). Phase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkers. Journal of Clinical Oncology, 27, 4986–4993.
James, N., Sydes, M., Clarke, N., Mason, M., Dearnaley, D., Anderson, J., Popert, R., Sanders, K., Morgan, R., Stansfeld, J., Dwyer, J., Masters, J., & Parmar, M. (2009). Systemic therapy for advancing or metastatic prostate cancer (STAMPEDE): a multi-arm, multistage randomized controlled trial. BJU., 103, 464–469.
Jendrossek, V. (2013). Targeting apoptosis pathways by Celecoxib in cancer. Cancer Letters, 332, 313–324.
Pruthi, R. S., & Wallen, E. M. (2005). Cyclooxygenase-2: a therapeutic target for prostate cancer. Clinical Genitourinary Cancer, 4, 203–211.
Sooriakumaran, P., Langley, S. E., Laing, R. W., & Coley, H. M. (2007). COX-2 inhibition: a possible role in the management of prostate cancer? Journal of Chemotherapy., 19, 21–32.
www.Clinicaltrials.gov. (2011) Effects of ASA on prostate tissue NCT00234299
www.Clinicaltrials.gov. (2013) Dexamethasone, aspirin, and diethylstilbestrol in treating patients with locally advanced or metastatic prostate cancer NCT00316927
de Groot, D. J., de Vries, E. G., Groen, H. J., & de Jong, S. (2007). Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Critical Reviews in Oncology/Hematology., 61, 52–69.
Ulrich, C. M., Bigler, J., & Potter, J. D. (2006). Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nature Reviews., 6, 130–140.
Robak, P., Smolewski, P., & Robak, T. (2008). The role of non-steroidal anti-inflammatory drugs in the risk of development and treatment of hematologic malignancies. Leukemia & Lymphoma., 49, 1452–1462.
Kashiwagi, E., Shiota, M., Yokomizo, A., Itsumi, M., Inokuchi, J., Uchiumi, T., & Naito, S. (2013). Prostaglandin receptor EP3 mediates growth inhibitory effect of aspirin through androgen receptor and contributes to castration resistance in prostate cancer cells. Endocrine-Related Cancer, 20, 431–441.
Bishr, M., & Saad, F. (2013). Overview of the latest treatments for castration-resistant prostate cancer. Nature reviews Urology, 10, 522–528.
Duque, G. (2008). Bone and fat connection in aging bone. Current Opinion in Rheumatology., 20, 429–434.
Nuttall, M. E., & Gimble, J. M. (2004). Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Current Opinion in Pharmacology., 4, 290–294.
Loberg, R., Ying, C., Craig, M., Day, L., Sargent, E., Neeley, C., Wojno, K., Snyder, L., Yan, L., & Pienta, K. (2007). Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Research, 67, 9417–9424.
Sandhu, S., Papadopoulos, K., Fong, P., Patnaik, A., Messiou, C., Olmos, D., Wang, G., Tromp, B., Puchalski, T., Balkwill, F., Berns, B., Seetharam, S., de Bono, J., & Tolcher, A. (2013). A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemotherapy and Pharmacology, 71, 1041–1050.
www.Clinicaltrials.gov. (2013) An open-label, multicenter, phase 2 study of single-agent CNTO 888 (an anti-CCL2 monoclonal antibody) for the treatment of subjects with metastatic castrate-resistant prostate cancer. NCT00992186
www.Clinicaltrials.gov. (2012) S0916, MLN1202 in treating patients with bone metastases. NCT01015560
Zheng, X., Cui, X., Avila, G., Huang, M., Liu, Y., Patel, J., Kong, A., Paulino, R., Shih, W., Lin, Y., Rabson, A., Reddy, B., & Conney, A. (2007). Atorvastatin and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clinical Cancer Research, 13, 5480–5487.
www.Clinicaltrials.gov. (2013) COX-2 inhibitor reduces serum PSA levels might predict a lower risk of prostatic cancer in men with LUTS/BPH with an elevated PSA level. NCT01678313
www.Clinicaltrials.gov. (2013) STAMPEDE: systemic therapy in advancing or metastatic prostate cancer: evaluation of drug efficacy: a multi-stage multi-arm randomised controlled trial. NCT00268476
www.Clinicaltrials.gov. (2013) A trial of COX-2 inhibitors in PSA recurrence after definitive radiation or radical prostatectomy for prostate cancer. NCT00073970
Singer, E., Palapattu, G., & van Wijngaarden, E. (2008). Prostate-specific antigen levels in relation to consumption of nonsteroidal anti-inflammatory drugs and acetaminophen: results from the 2001-2002 National Health and Nutrition Examination Survey. Cancer, 113, 2053–2057.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hardaway, A.L., Herroon, M.K., Rajagurubandara, E. et al. Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev 33, 527–543 (2014). https://doi.org/10.1007/s10555-013-9484-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9484-y