Abstract
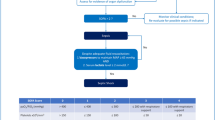
Sepsis is a serious organ dysfunction leading to endothelial damage in critical patients. Physiologically, there is an augment of vascular diameter in response to increased vascular blood flow and shear stress stimulus. However, the pattern of vascular response in face of passive mobilization (PM), an early mobilization physical strategy, has not yet been explored in patients with sepsis. To explore patterns of vascular response to PM and associations with clinical and cardiovascular profile in patients with sepsis. Cross-sectional, single-arm study. Thirty-two patients diagnosed with sepsis were enrolled. Vascular response was assessed by flow-mediated dilation (FMD) using brachial artery ultrasound, before and after PM. The PM (to assess the response pattern) and SR (shear rate) were also calculated. PM protocol consisted of knees, hips, wrists, elbows, shoulders, dorsiflexion/plantar flexion movements 3 × 10 repetitions each (15 min). Arterial stiffness was assessed by Sphygmocor®, by analyzing the morphology and pulse wave velocity. Cardiac autonomic modulation (CAM) was assessed by analyzing heart rate variability indexes (mean HR, RMSSD, LF, HF, ApEn, SampEn, DFA). Different vascular responses were observed after PM: (1) increased vascular diameter (responders) (n = 13, %FMD = 11.89 ± 5.64) and (2) reduced vascular diameter (non-responders) (n = 19, %FMD= −7.42 ± 6.44). Responders presented a higher non-linear DFA2 index (p = 0.02). There was a positive association between FMD and DFA (r = 0.529; p = 0.03); FMD and SampEn (r = 0.633; p < 0.01). A negative association was identified between FMD and LF (Hz) (r= −0.680; p < 0.01) and IL-6 (r= −0.469; p = 0.037) and SR and CRP (r= −0.427; p = 0.03).






Similar content being viewed by others
References
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR et al (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395:200–211. https://doi.org/10.1016/S0140-6736(19)32989-7
Vender JS, Szokol JW, Murphy GS, Nitsun M (2004) Sedation, analgesia, and neuromuscular blockade in sepsis: An evidence-based review. Crit Care Med 32(11):S554–S561. https://doi.org/10.1097/01.CCM.0000145907.86298.12
Grunow JJ, Goll M, Carbon NM, Liebl ME, Weber-Carstens S, Wollersheim T (2019) Differential contractile response of critically ill patients to neuromuscular electrical stimulation. Critical Care 23(1):1–12. https://doi.org/10.1186/s13054-019-2540-4
Conceição MS, Ugrinowitsch C 2019 Exercise with blood flow restriction: an effective alternative for the non-pharmaceutical treatment for muscle wasting. Journal of Cachexia, Sarcopenia and Muscle. Wiley Blackwell, pp 257–262. https://doi.org/10.1002/jcsm.12397
Koo NYK, Choong K, Fan E (2011) Prioritizing rehabilitation strategies in the care of the critically Ill. Critical Care Rounds 8(4):1:7
Trinity JD, Richardson RS (2019) Physiological Impact and Clinical Relevance of Passive Exercise/Movement. Sports Medicine. Springer International Publishing 49(9):1365–1381. https://doi.org/10.1007/s40279-019-01146-1
Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascón GA et al (2016) The endothelium in sepsis. Shock Lippincott Williams Wilkins 45(3):259–270. https://doi.org/10.1097/SHK.0000000000000473
Nelson AD, Rossman MJ, Witman MA, Barrett-O’Keefe Z, Groot HJ, Garten RS et al (2016) Nitric oxide-mediated vascular function in sepsis using passive leg movement as a novel assessment: A cross-sectional study. J Appl Physiol 120:991–999. https://doi.org/10.1152/japplphysiol.00961.2015
Joffre J, Hellman J, Ince C, Ait-Oufella H (2020) Endothelial responses in sepsis. American Journal of Respiratory Critical Care Medicine American Thoracic Society 202(3):361–370. https://doi.org/10.1164/rccm.201910-1911TR
Phillips SA et al (2017) Exploring Vascular Function Biomarkers Implications for Rehabilitation. Braz J Cardiovasc Surg. 32(2):125–135. https://doi.org/10.21470/1678-9741-2016-0085
Hsiao SY et al (2018) Concentration and value of endocan on outcome in adult patients after severe sepsis. Clinica Chimica Acta 483:275–280. https://doi.org/10.1016/j.cca.2018.05.007
Kung CT et al (2013) Serum adhesion molecules as predictors of bacteremia in adult severe sepsis patients at the emergency department. Clin Chim Acta 421(5):116–120. https://doi.org/10.1016/j.cca.2013.02.023
Bruno RM, Ghiadoni L, Seravalle G, Dell’oro R, Taddei S, Grassi G (2012) Sympathetic regulation of vascular function in health and disease. Front Physiol 3:284. https://doi.org/10.3389/fphys.2012.00284
Millar PJ, Notarius CF, Haruki N, Floras JS (2019) Heart failure–specific relationship between muscle sympathetic nerve activity and aortic wave reflection. J Card Fail 25:404–408. https://doi.org/10.1016/j.cardfail.2019. 03.005
Carrara M, Herpain A, Baselli G, Ferrario M (2020) Vascular Decoupling in Septic Shock: The Combined Role of Autonomic Nervous System, Arterial Stiffness, and Peripheral Vascular Tone. Front Physiol 11:594. https://doi.org/10.3389/fphys.2020.00594
Singer M et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA - J Am Med Assoc 315(8):801–810. https://doi.org/10.1001/jama.2016.0287
Pinheiro TT, de Freitas FGR, Coimbra KTF, Mendez VMF, Rossetti HB, Talma PV et al (2017) Short-term effects of passive mobilization on the sublingual microcirculation and on the systemic circulation in patients with septic shock. Ann Intensive Care. (1):95. https://doi.org/10.1186/s13613-017-0318-x
Hallmark R, Patrie JT, Liu Z, Gaesser GA, Barrett EJ, Weltman A (2014) The effect of exercise intensity on endothelial function in physically inactive lean and obese adults. PLoS One 9(1):e85450. https://doi.org/10.1371/journal.pone.0085450
Thijssen DHJ et al (2019) Expert consensus and evidence-based recommendations for the assessment of flowmediated dilation in humans. Eur Heart J 40: 2534–2547. https://doi.org/10.1093/eurheartj/ehz350
Radespiel-Troger M, Rauh R, Mahlke C, Gottschalk T, Muck-Weymann M (2003) Agreement of two different methods for measurement of heart rate variability. Clin Auton Res 13:99–102
Vanderlei LCM et al. (2009) Noções básicas da variabilidade da FC e sua aplicabilidade clínica. Revista Brasileira de Cirurgia Cardiovascular, v. 24, n. 2, p. 205–217
Doupis J, Papanas N, Cohen A, McFarlan L, Horton E (2016) Pulse Wave Analysis by Applanation Tonometry for the Measurement of Arterial Stiffness. Open Cardiovasc Med J 10:188–195. https://doi.org/10.2174/1874192401610010188
Dantas CM et al (2012) Influência da mobilização precoce na força muscular periférica e respiratória em pacientes críticos. RevBrasTerIntensiva v 24(2):173–178
Langer D et al (2009) Early exercise in critically ill patients enhances short-term functional recovery*. Crit Care Med v 37:9, p. 2499–2505 n.
Munro BH (2001) Correlation. In: Munro BH. Statistical methods for health care research, 4ª. Lippincott, ed. Philadelphia, pp 223–243
Becker L et al (2012) Endothelial dysfunction assessed by brachial artery ultrasound in severe sepsis and septic shock. J Critical Care. 27(3): 316.e9-316.https://doi.org/10.1016/j.jcrc.2011.08.002
Hoiland RL, Tremblay JC, Stacey BS, Coombs GB, Nowak-Flück D, Tymko MM, Patrician A, Stembridge M, Howe CA, Bailey DM, Green DJ, MacLeod DB, Ainslie PN (2020) Acute reductions in haematocrit increase flow-mediated dilatation independent of resting nitric oxide bioavailability in humans. J Physiol 598(19):4225–4236. https://doi.org/10.1113/JP280141
Dawson EA, Green DJ, Cable NT, Thijssen DH (2013) Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol (1985) 115(11):1589–1598. https://doi.org/10.1152/japplphysiol.00450.2013
Ichinose M, Nakabayashi M, Ono Y (2018) Sympathoexcitation constrains vasodilation in the human skeletal muscle microvasculature during postocclusive reactive hyperemia. Am J Physiol Heart Circ Physiol. 1;315(2):H242-H253. https://doi.org/10.1152/ajpheart.00010.2018. Epub 2018 Apr 13. PMID: 29652542
Hein TW, Singh U, Vasquez-Vivar J, Devaraj S, Kuo L, Jialal I (2009) Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis 206(1):61–68. https://doi.org/10.1016/j.atherosclerosis.2009.02.002
Schwedler SB, Kuhlencordt PJ, Ponnuswamy PP et al (2007) Native C-reactive protein induces endothelial dysfunction in ApoE-/- mice: implications for iNOS and reactive oxygen species. Atherosclerosis 195(2):e76. https://doi.org/10.1016/j.atherosclerosis.2007.06.013
Ramos AM, Pellanda LC, Gus I, Portal VL (2009) Inflammatory markers of cardiovascular disease in the elderly. Arq Bras Cardiol 92(3):221–228. https://doi.org/10.1590/s0066-782X2009000300012
Teixeira BC, Lopes AL, Macedo RCO, Correa CS, Ramis TR, Ribeiro JL, Reischak-Oliveira A (2014) Inflammatory markers, endothelial function and cardiovascular risk. J vasc bras 13(2):108:115. https://doi.org/10.1590/jvb.2014.054
Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB (1993) Distribution and characterization of tumor necrosis factor-alpha-like immunoreactivity in the murine central nervous system. J Comp Neurol 22(4):543–567 337(
Wexler O, Morgan MA, Gough MS, Steinmetz SD, Mack CM, Darling DC, Doolin KP, Apostolakos MJ, Graves BT, Frampton MW, Chen X, Pietropaoli AP (2012) Brachial artery reactivity in patients with severe sepsis: an observational study. Crit Care 16(2):38. https://doi.org/10.1186/cc11223
de Souza AC, Cisternas JR, de Abreu LC, Roque AL, Monteiro CB, Adami F, Vanderlei LC, Sousa FH, Ferreira LL, Valenti VE (2014) Fractal correlation property of heart rate variability in response to the postural change maneuver in healthy women. Int Arch Med 7(1):1–7. https://doi.org/10.1186/1755-7682-7-25
Bonjorno Junior JC, Caruso FR, Mendes RG, da Silva TR, Biazon TMPC, Rangel F, Phillips SA, Arena R, Borghi-Silva A (2019) Noninvasive measurements of hemodynamic, autonomic and endothelial function as predictors of mortality in sepsis: A prospective cohort study. PLoS One. 11;14(3). https://doi.org/10.1371/journal.pone.0213239
Fiskum C, Andersen TG, Bornas X, Aslaksen PM, Flaten MA, Jacobsen K (2018) Non-linear Heart Rate Variability as a Discriminator of Internalizing Psychopathology and Negative Affect in Children With Internalizing Problems and Healthy Controls. Front Physiol 9:561. https://doi.org/10.3389/fphys.2018.00561
Acknowledgements
We would like to thank the professionals from Fraternity of the Santa Casa Misericordia Hospital of São Carlos for their support with data collection.
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil [grant number 428073/2016-6], financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001 and FAPESP [grant number 2015/26501-1].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Débora Mayumi de Oliveira Kawakami, Tamara Rodrigues da Silva Destro, Thaís Marina Pires de Campos Biazon, Naiara Molina Garcia and Flávia Cristina Rossi Caruso Bonjorno. The first draft of the manuscript was written by Débora Mayumi de Oliveira Kawakami, José Carlos Bonjorno-Junior, Audrey Borgh-Silva and Renata Gonçalves Mendes and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee [University of Sao Carlos (CAAE: 58405916.4.0000.5504, protocol number: 2.363.397)]. and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawakami, D.M.d.O., Bonjorno-Junior, J.C., da Silva Destro, T.R. et al. Patterns of vascular response immediately after passive mobilization in patients with sepsis: an observational transversal study. Int J Cardiovasc Imaging 38, 297–308 (2022). https://doi.org/10.1007/s10554-021-02402-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02402-0