Abstract
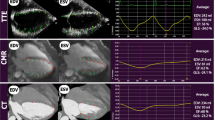
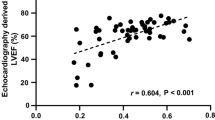
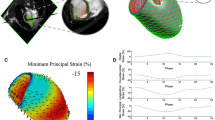
We assessed CT-derived left ventricular strain in a cohort of patients referred for transcatheter aortic valve implantation (TAVI) and validated it against 2 dimensional speckle tracking echocardiography as the gold standard. 65 consecutive patients with symptomatic aortic valve stenosis referred for CT imaging prior to TAVI were included in this analysis. For all patients, retrospectively ECG-gated multi-phase functional CT data sets acquired with identical reconstruction parameters were available. All data sets were acquired using a third generation dual source system. In all patients, multiphase reconstructions in increments of 10% of the cardiac cycle were rendered (slice thickness 0.75, increment 0.5 mm, medium smooth reconstruction kernel) and transferred to a dedicated workstation (Ziostation2, Ziosoft Inc., Tokyo, Japan). Additional functional reconstructions for dynamic assessment and quantification of strain were processed. Multiplanar reconstructions (MPR) of the left ventricle similar to standard echocardiographic 4, 2 and apical 3 chamber views were rendered in CT. Similar to echocardiographic longitudinal strain, the perimeter of the left ventricle was manually traced within the myocardium and peak maximal shortening as a parameter representing longitudinal strain was calculated for each view and averaged to obtain a marker for global longitudinal strain (CT perimeter-derived strain). Furthermore, for quantification of 3-dimensional strain, endocardial and epicardial borders of myocardium were marked in six short axis views and peak maximum 3- dimensional strain of the myocardium was calculated in standard six basal, six mid and four apical segments. 3-dimensional strain values of the 16 standard segments as well as perimeter-derived strain values in the three standard windows were averaged to obtain global strain. Echocardiography was performed in all patients before CT data acquisition. Digital loops were acquired from three apical views (four-, two-, and three chamber views). For assessment of 2 dimensional global longitudinal strain (GLS), recordings were processed with acoustic-tracking software allowing offline semiautomated speckle-based strain analyses. The mean age of all 65 patients was 81 ± 5 years. The mean echocardiographic ejection fraction and mean echocardiographic GLS were 50 ± 12% and −13.6 ± 4.5%, respectively. The mean CT-derived peak 3-dimensional global strain and mean peak strain derived by perimeter was 43.2 ± 13.5% and −11.2 ± 3.5%, respectively. Both CTderived global 3D-strain and perimeter derived strain showed a significant correlation to GLS derived by echocardiography (r = −0.8, p < 0.0001 for 3D strain and r = 0.71, p < 0.0001 for perimeter-derived strain). Bland-Altman analysis showed a systematic underestimation (i. e. worse strain values) of CT perimeter-derived strain compared to GLS by echocardiography (mean difference −2.4% with 95% limits of agreement between 4% to −9%). ROC Curve analysis assuming a normal GLS when less than −18% showed that a CT-derived peak 3-dimensional global strain cut-off-value of 45% has a sensitivity of 91% and a specificity of 60% for detecting normal left ventricular strain (AUC 0.81, p = 0.001). For CT perimeter-derived strain, a cut-off value of −12%—assuming a normal echocardiographic GLS when less than −18%—achieved a sensitivity of 82% and a specificity of 61% (AUC of 0.82, p = 0.001) for detecting abnormal left ventricular strain. Using dedicated software, assessment of CT-derived left ventricular strain is feasible and comparable to strain derived by echocardiographic 2 dimensional speckle tracking.





Similar content being viewed by others
References
Iacoviello M, Puzzovivo A, Guida P, Forleo C, Monitillo F, Catanzaro R et al (2013) Independent role of left ventricular global longitudinal strain in predicting prognosis of chronic heart failure patients. Echocardiography 30(7):803–811
Mentias A, Naji P, Gillinov AM, Rodriguez LL, Reed G, Mihaljevic T et al (2016) Strain Echocardiography and Functional Capacity in Asymptomatic Primary Mitral Regurgitation With Preserved Ejection Fraction. J Am Coll Cardiol 68(18):1974–1986
Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD et al (2012) Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol 60(20):2074–2081
Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL et al (2014) Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail 16(12):1301–1309
Zhang KW, French B, May Khan A, Plappert T, Fang JC, Sweitzer NK et al (2014) Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. J Am Heart Assoc 3(1):e000550
Bansal M, Kasliwal RR (2013) How do I do it? Speckle-tracking echocardiography. Indian Heart J 65(1):117–123
D’Ascenzi F, Cameli M, Iadanza A, Lisi M, Zaca V, Reccia R et al (2013) Improvement of left ventricular longitudinal systolic function after transcatheter aortic valve implantation: a speckle-tracking prospective study. Int J Cardiovasc Imaging 29(5):1007–1015
Spethmann S, Dreger H, Baldenhofer G, Stuer K, Saghabalyan D, Muller E et al (2013) Short-term effects of transcatheter aortic valve implantation on left atrial mechanics and left ventricular diastolic function. J Am Soc Echocardiogr 26(1):64–71
Benyounes N, Lang S, Soulat-Dufour L, Obadia M, Gout O, Chevalier G et al (2015) Can global longitudinal strain predict reduced left ventricular ejection fraction in daily echocardiographic practice? Arch Cardiovasc Dis 108(1):50–56
Yamamuro M, Tadamura E, Kubo S, Toyoda H, Nishina T, Ohba M et al (2005) Cardiac functional analysis with multi-detector row CT and segmental reconstruction algorithm: comparison with echocardiography, SPECT, and MR imaging. Radiology 234(2):381–390
Marwan M, Ammon F, Bittner D, Rother J, Mekkhala N, Hell M et al (2018) CT-derived left ventricular global strain in aortic valve stenosis patients: a comparative analysis pre and post transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr 12(3):240–244
Tavakoli V, Sahba N (2014) Assessment of subendocardial vs. subepicardial left ventricular twist using tagged MRI images. Cardiovasc Diagn Ther 4(2):56–63
Tavakoli V, Sahba N (2014) Cardiac motion and strain detection using 4D CT images: comparison with tagged MRI, and echocardiography. Int J Cardiovasc Imaging 30(1):175–184
Masri A, Schoenhagen P, Svensson L, Kapadia SR, Griffin BP, Tuzcu EM et al (2014) Dynamic characterization of aortic annulus geometry and morphology with multimodality imaging: predictive value for aortic regurgitation after transcatheter aortic valve replacement. J Thorac Cardiovasc Surg 147(6):1847–1854
Tanabe Y, Kido T, Kurata A, Sawada S, Suekuni H, Kido T et al (2017) Three-dimensional maximum principal strain using cardiac computed tomography for identification of myocardial infarction. Eur Radiol 27(4):1667–1675
Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH (2013) Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 26(2):185–191
Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P et al (2009) Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging 2(1):80–84
Blessberger H, Binder T (2010) NON-invasive imaging: two dimensional speckle tracking echocardiography: basic principles. Heart 96(9):716–722
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G et al (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: aSE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr 24(3):277–313
Sengupta PP, Krishnamoorthy VK, Korinek J, Narula J, Vannan MA, Lester SJ et al (2007) Left ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr 20(5):539–551
Tee MW, Won S, Raman FS, Yi C, Vigneault DM, Davies-Venn C et al (2015) Regional Strain Analysis with Multidetector CT in a Swine Cardiomyopathy Model: relationship to Cardiac MR Tagging and Myocardial Fibrosis. Radiology 277(1):88–94
Helle-Valle TM, Yu WC, Fernandes VR, Rosen BD, Lima JA (2010) Usefulness of radial strain mapping by multidetector computer tomography to quantify regional myocardial function in patients with healed myocardial infarction. Am J Cardiol 106(4):483–491
Miyazaki S, Daimon M, Miyazaki T, Onishi Y, Koiso Y, Nishizaki Y et al (2011) Global longitudinal strain in relation to the severity of aortic stenosis: a two-dimensional speckle-tracking study. Echocardiography 28(7):703–708
Ng ACT, Prihadi EA, Antoni ML, Bertini M, Ewe SH, Ajmone Marsan N et al (2017) Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur Heart J Cardiovasc Imaging 19(8):859–867
Acknowledgements
We would like to express our sincere thanks to Mr. Tsuyoshi Nagata from Ziosoft for his help and assistance in the pre-processing and analysis of the strain data as well as his continuous support with data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mohamed Marwan has received speaker honoraria from Siemens Healthineers and Edwards Lifesciences. Martin Arnold is a consultant for St. Jude Medical and Edwards Lifesciences. The other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ammon, F., Bittner, D., Hell, M. et al. CT-derived left ventricular global strain: a head-to-head comparison with speckle tracking echocardiography. Int J Cardiovasc Imaging 35, 1701–1707 (2019). https://doi.org/10.1007/s10554-019-01596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01596-8