Abstract
We studied the dynamic change of high-risk plaque detected by coronary computed tomographic angiography (CCTA) in patients with subclinical coronary artery disease. We analyzed paired CCTA findings in 103 patients. The follow-up period was 1.37 ± 0.49 years. We studied the prevalence and change of high-risk plaque (HRP) defined as positive remodeling, low attenuation plaque, spotty calcification, or napkin-ring sign. Of 413 plaques detected by CCTA, thirty-seven patients had 47 HRP at baseline. At follow-up, eight patients showed disappearance of HRP. Sixty-six patients had no HRP at baseline. At follow-up, new HRP was detected in 12 patients. As a whole, 20 patients (19.4 %) showed appearance or disappearance of HRP. For 29 patients with persistent HRP, five of twenty-one patients with one HRP at baseline showed two HRP at follow-up. Five of eight patients with two HRP at baseline showed one HRP at follow-up. As a whole, in 27 % of patients with persistent HRP, the number of HRP changed. These changes were neither associated with statin use nor location of the HRP. Our results showed that about 20 % of patients showed appearance or disappearance of HRP 1 year later. In addition, in 27 % of patients with persistent HRP, the number of HRP increased or decreased. These results suggest a dynamic nature of HRP.
Similar content being viewed by others
Introduction
Pathologic studies have shown that coronary artery disease (CAD) is an active process that is likely to constantly changing [1, 2]. Imaging studies also suggest that atherosclerosis is a diffuse and dynamic process with plaque undergoing biological remodeling and compositional alterations [3]. Previous studies demonstrate that 70–80 % of cardiac death and myocardial infarction are caused by rupture of the vulnerable plaque, which has often non-significant stenosis [4, 5]. Therefore, the identification of vulnerable plaque is important. However, recent studies using intravascular ultrasound showed that vulnerability of coronary plaques changed dynamically in a relatively short period [6, 7].
Coronary computed tomographic angiography (CCTA) is a promising modality to evaluate CAD. CCTA has an excellent capacity to detect coronary stenosis and vulnerable plaque. In well-performed, prospective multicenter studies, CCTA is an accurate diagnostic modality with superior accuracy to detect and exclude anatomically obstructive CAD [8–10]. In addition, CCTA can non-invasively characterize high-risk vulnerable plaque and can predict future acute coronary syndrome (ACS) event in patients with high-risk plaques (HRP) [11–13]. Furthermore, CCTA can non-invasively and serially evaluate the changes of coronary plaques. CCTA can evaluate all coronary trees, including distal part of main coronary branches and side branches. Thus, our aim is to study the changes of vulnerable plaque detected by CCTA and related factors of these changes in patients with subclinical CAD.
Methods
Patients
From April 2011 through December 2015, we performed CCTA in 930 patients. Figure 1 shows the flow chart of studied patients. The following patients were excluded; patients with ACS (ACS, n = 82), patients who underwent coronary revascularization (n = 74), patients with known CAD (n = 178), and patients with inadequate image quality because of motion artifacts, blooming artifacts, or severe calcification (n = 46), leaving 550 patients. Most patients were referred to our hospital for the evaluation of CAD because of multiple risk factors and/or symptom of chest pain.
We divided these patients into the three groups; patients with HRP (n = 45), patients without HRP but with coronary calcium score (CCS) ≥ 400 (n = 82), and patients without HRP and CCS <400 (n = 423). For patients with HRP or CCS >400, we prescribed high-intensity statin therapy to achieve LDL-C level of 70–80 mg/dl, at least <100 mg/dl. For these patients, we planned follow-up CCTA to monitor the progression or regression of subclinical CAD 1 year after the start of statin therapy. This is a prospective study.
Follow-up CCTA was performed in 37 patients (82.2 %) of those with HRP and in 66 patients (80.5 %) with no HRP but CCS >400. Thus, we analyzed paired CCTA findings of 103 patients. Follow-up period was 1.37 ± 0.49 years.
Coronary computed tomographic angiography (CCTA)
We scanned all patients with a 64 multi-detector computed tomography (MDCT) scanner (SOMATOM Sensation 64 Cardiac, Siemens Medical Solutions, Erlangen, Germany). We administered metoprolol 20 mg orally before the 64-MDCT scan in patients with a heart rate >70beats/min. We also administered sublingual nitroglycerin 0.8 mg before the scan to achieve maximal coronary vasodilation.
We acquired contrast-enhanced CT angiography data with the use of a spiral scan with 32 × 0.6-mm collimation, 330-ms gantry rotation, pitch of 0.2, and tube voltage at 120 kV. We acquired a total of 64 overlapping 0.6-mm slices per rotation with the use of a focal spot periodically moving in the longitudinal direction. We modulated tube current according to the ECG, with a maximum current of 850–950 mAs during a time period of approximately 330 ms centered at 375 ms before the next R-wave and reduction by 80 % during the remaining cardiac cycle. We injected contrast agent (60–70 ml; 370 mg iodine/ml) intravenously (4.0 ml/s) followed by a 30-ml saline chaser. We reconstructed transaxial images with the use of an ECG-gated half-scan reconstruction algorithm and kernel B30f. As for the phase of cardiac cycle, we measured the msec after the R wave. We did not use percentages.
CCTA image interpretation
We transferred CT data sets to an offline workstation (Aquarius NetStation, Terarecon In, San Meteo, CA, USA) for image analysis. The contrast-enhanced CT angiography data were evaluated by two reviewers, who were blinded to the clinical characteristics of studied patients, with maximum intensity and curved multiplanar reconstruction techniques along multiple longitudinal axes and transversely. Standard display settings were used for the evaluation of the contrast-enhanced 64-MDCT scans (window width 800 Hounsfield units; window center 250 HU). We reviewed paired CCTA images of each patient. We identified each plaque in the first CCTA image and checked the change of the same plaque in the follow-up CCTA image. Analysis was made in the cross-section with the worst plaque burden.
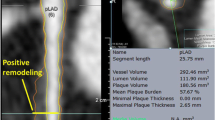
HRP was defined as plaque with positive remodeling, low attenuation, spotty calcification, or a napkin-ring sign. Positive remodeling was defined as a diameter at the plaque site more than 10 % larger than that of a reference segment [11]. Low-attenuation plaque was defined as the lowest CT number less than 30 Hounsfield units (HU) in non-calcified plaque or plaque with spotty calcification [11]. Spotty calcification was defined as a calcification <3 mm in size on curved multiplanar reformation images and occupies only one side on cross-sectional images [11]. A napkin-ring sign was defined as the presence of a ring of high attenuation around certain coronary artery plaque, with CT attenuation of the ring presenting higher than that of adjacent plaque but no greater than 130 HU [12].
We divided our patients into four groups. Persistent HRP group was defined as patients in whom HRP was present at both baseline and follow-up (n = 29). Disappeared HRP group was defined as patients whose HRP at baseline disappeared at follow-up (n = 8). New HRP group was defined as patients in whom new HRP was detected at follow-up (n = 12). No HRP group was defined as patients in whom no HRP was detected at baseline and follow-up (n = 54).
Two reviewers identified the location of each HRP according to a modified American Heart Association classification [14]. We defined proximal location as segment 1, 5, 6 and 11 and distal location as segment 2, 3, 7, 8, 12, and 13.
This study was reviewed and approved by the Institutional Review Board of Okayama Kyokuto Hospital. All study participants provided informed consent prior to study enrollment. The authors declare that there is no conflict of interest.
Statistical analysis
Data are expressed as mean ± SD. Continuous variables were compared with two group t test between the two groups and by one-factor ANOVA among the four groups. Discrete variables were expressed as counts or percentage and compared with Chi square or the Fisher’s exact test between the two groups and Chi-aquare test for independence among the three or four groups. A p-value <0.05 was considered statistically significant.
Results
Clinical characteristics of the studied patients are shown in Table 1. Figure 2 shows the changes of the HRP. Thirty-seven patients had HRP at baseline. At follow-up CCTA, eight patients (21.6 %) showed disappearance of HRP. Sixty-six patients had no HRP at baseline. At follow-up CCTA, 12 patients (18.2 %) showed new HRP. As a whole, 20 patients (19.4 %) showed appearance or disappearance of HRP.
Table 2 shows the changes of the number of all plaques and HRP. As a whole, the prevalence of HRP was not significantly different between at baseline and follow-up. Also in persistent HRP group, the prevalence of HRP was not significantly different between at baseline and follow-up. Figure 3 shows the changes of the number of HRP in persistent HRP group. Twenty-one patients showed one HRP at baseline, but five patients showed two HRP at follow-up. On the other hand, eight patients showed two HRP at baseline, but five patients showed one HRP at follow-up. As a whole, the number of HRP changed in 10 patients (27.0 %).
Table 3 showed the changes of LDL-C value among the four groups. In each group, LDL-C level significantly decreased from baseline to follow-up period except the appeared HRP group. However, there were no significant differences among the four groups both at baseline and follow-up. Table 4 showed the location of HRP. The percentage of proximal lesion was not significantly different among the three groups. Table 5 showed the studies which investigated the dynamic changes of coronary plaques. Figure 4 showed the representative cases of disappeared HRP and new HRP.
Representative cases of disappeared HRP and appeared HRP. a High-risk plaque in the proximal portion of right coronary artery disappeared after 1.1 year high-intensity statin therapy in a 70 year-old man. His LDL-C level decreased from 153 to 80 mg/dl. b High-risk plaque in the mid-portion of left anterior descending coronary artery appeared despite 1.2 year high-intensity statin therapy in a 65 year-old man. His LDL-C level was kept between 71 and 93 mg/dl. HRP high-risk plaque, LDL-C low-density lipoprotein cholesterol
Discussion
Our results showed that 20 % of patients showed appearance or disappearance of HRP at follow-up. This dynamic nature of HRP was observed during high-intensity statin therapy. Also, in 27 % of patients with persistent HRP, the number of HRP increased or decreased. These results suggest a dynamic nature of HRP detected by CCTA.
High risk plaque (HRP)
Recent studies demonstrate that CCTA characteristics of plaques associated with vulnerable plaque are positive remodeling, low attenuation plaque, and spotty calcification [11]. In addition, napkin-ring sign appears as a sign of high-risk coronary plaque [12]. Thus, we defined HRP as positive remodeling, low attenuation plaque, spotty calcification, or napkin ring sign.
Thomsen et al. performed a systematic review and meta-analysis to study the characteristics of HRP and its association of prognosis [13]. ACS patients had significantly higher number of non-calcified plaque and spotty calcified plaque compared with stable angina (SA) patients. Remodeling index was higher in culprit lesions in ACS compared with SA and compared with non-culprit lesions in ACS patients. The associated risk of future ACS was significantly higher in high-risk than in low-risk plaques. Thus, CCTA can non-invasively characterize high-risk vulnerable coronary plaques and can predict future ACS events in patients with HRPs.
Dynamic changes of HRP and related factors
Our results also showed that not only the number of patients with HRP changed dynamically, but also the number of HRP in a patient with persistent HRP changed dynamically. In our study, all patients received high-intensity statin therapy after the baseline CCTA. As shown in Table 3, LDL-C level was not significantly different among the four groups, although mean LDL-C level was less than 90 mg/dl. Thus, despite statin therapy, new HRP may appear or prior HRP regress. On the other hand, intravascular ultrasound studies have demonstrated that plaque regression is observed when LDL-C decreases below 70–80 mg/dl [15]. Thus, more intensive lowering of LDL-C below 70–80 mg/dl may be needed to prevent the progression of HRP.
Numerous pathological, angiographic, and imaging studies have shown a proximal predisposition of thin-cap fibroatheroma (TCFA), acute occlusions, or plaque ruptures [7, 16]. Kubo et al., using virtual histology intravascular ultrasound (VH-IVUS), showed that proximal TCFA in larger vessels with more plaque seemed to heal less often compared with distal TCFA and that this might contribute to plaque rupture and subsequent thrombosis [6]. Thus, we studied the relationship between the location of HRP and changes of HRP (Table 4). However, we found no significant differences of the location among persistent HRP group, disappeared HRP group, and appeared HRP group.
Comparison with previous studies
Our study showed the dynamic change of HRPs. There are few studies which investigated the dynamic nature of coronary artery lesion morphology (Table 5). Kubo et al. performed serial VH-IVUS studies and examined 216 nonculprit lesions in 99 patients [6]. At baseline, 20 lesions were VH-IVUS-derived TCFA (VH-TCFA)s; during follow-up, 15 (75 %) VH-TCFAs healed, 13 became thick-capped fibroatheroma (ThCFA)s, two became fibrotic plaque, and 5 (25 %) remained unchanged. Conversely, 12 new VH-TCFAs developed; six late-developing VH-TCFAs were pathological intimal thickening (PIT)s, and six were ThCFAs at baseline.
A serial IVUS analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial investigated 99 untreated nonculprit lesions in 63 patients with ST-elevation myocardial infarction [7]. Lesions were classified as PIT, TCFA, ThCFA, fibrotic plaque, or fibrocalcific plaque. The frequency of TCFA increased from 41 % at baseline to 54 % at follow-up, whereas ThCFAs decreased from 41 to 34 % and PIT decreased from 16–8 %. Among the 41 lesions classified at baseline as TCFA, at follow-up, 32 (78 %) were still classified as TCFA, whereas 9 (22 %) were classified as ThCFAs or fibrotic plaques. An additional 21 lesions at follow-up were newly classified as TCFA, developing from either PIT or ThCFA.
Motoyama et al. studied 3,150 patients who underwent CCTA and evaluated the association between plaque characteristics (HRP and significant stenosis[SS]) and mid-term prognosis [17]. ACS occurred more frequently in 294 HRP(+) patients (16.3 %) compared with 2864 HRP(−) patients (1.4 %). ACS was also significantly more frequent in SS(+) (5.5 %) than SS(−) patients (2.1 %). In 449 patients who underwent serial CCTA, HRP disappeared in 4 of 61 patients (6.6 %) with baseline HRP. On the other hand, HRP appeared in 15 of 347 patients (4.1 %) without baseline HRP.
Table 5 shows the results of these three studies and our study. The prevalence of appearance and disappearance of HRP varies widely among the four studies. Kubo’s study used VH-IVUS and Zhao’s study used IVUS for imaging, while those of Motoyama et al. and we used CCTA. Thus, the definition of HRP was different among the four groups. Follow-up period was relatively short except a study of Motoyama et al. Stability of CAD is likely an important factor for dynamic change of HRP. Kubo’s study included some ACS patients and all patients of Zhao’s study had ST-elevation myocardial infarction. On the other hand, all patients of two CCTA studies were stable CAD. The prevalence of disappearance of HRP was very high in Kubo’s study, while the other three studies showed the prevalence of 10–20 %. On the other hand, the prevalence of new HRP was high in Zhao’ study, while the other studies showed the prevalence of 5–20 %. In our study, the prevalence of appearance or disappearance of HRP was relatively high (about 20 %), although all patients had stable CAD. The fact that the prevalence of risk factors (hypertension, dyslipidemia, and diabetes) was highest among the four studies may account for some of these findings. Also, the prevalence of statin use and intensity of statin therapy seems to play some role. Thus, differences of imaging modality, stability of CAD, patient characteristics, statin usage, definition of HRP would explain some of these differences. On the other hand, these studies suggest that a plaque that appears vulnerable at one time may be less vulnerable in a relatively short period, whereas another plaque, initially not vulnerable, may have developed vulnerable characteristics within the same time frame.
Limitations for the evaluation of calcified and non-calcified plaques
Recent meta-analysis showed that compared with IVUS, CCTA was highly accurate for estimation of lumen area, percentage of area stenosis, plaque volume, and plaque area and for detection of plaque [18]. For plaque characterization, it has been shown that CT-derived attenuation values are different in calcified, fibrous, and lipid rich plaques [19]. Using VH-IVUS as reference standard, one study showed that 64-slice CCTA underestimated non-calcified plaque volume by 17 % and overestimated mixed/calcified plaque volume by 3 % [20]. The other study showed that dual-source 64-slice CT overestimated calcified and lipid-rich components by 2.2 and 3.7 %, respectively, and underestimated fatty plaque component by 5.6 % [21]. In ATLANTA I study, lumen, vessel, non-calcified, and calcified-plaque areas were overestimated on CCTA by 22, 19, 44, and 88 %, respectively, on a slice-by-slice basis. It also showed that high-density non-calcified plaque best correlated with fibrous tissue, and that low-density non-calcified plaque correlated with necrotic core plus fibrofatty tissue by VH-IVUS [22].
Clinical implications
Thus, identifying an individual plaque may not provide the right answer. Because of the temporal relationship of its vulnerable characteristics, identifying a single HRP is unlikely to be of incremental benefit for risk prediction over established factors. Our focus should remain on the entire atherosclerotic process and prevention of diffuse disease. The detection of a state of vulnerability in a patient seems to be more important than detection of individual sites of vulnerability.
Limitations
There are some limitations in our study. First, there is a selection bias of patients, because not all the patients underwent serial CT scans and follow-up rate was 81.1 %. Other patients declined follow-up CCTA, mainly because of no symptoms. Second, there might be some patients who showed disappearance of HRP but appearance of new HRP. We had no classification for these patients. However, we did not observe these patients in our study. Third, the number of patients is relatively small. However, our point is that not negligible number of patients experience either appearance or disappearance of HRP. Fourth, definition of HRP detected by CCTA is not established. Different definition would likely to lead a different prevalence of HRP changes. Fifth, our study did not fully elucidate its mechanistic aspect. Although we investigated the association of LDL-C value and location of HRP with dynamic change of HRP, we did not measure inflammatory markers, such as high sensitivity CRP, or other lipid panels. Thus, further study is needed to evaluate the factors associated with changes of HRP.
Conclusions
Our results showed that 20 % of patients experience either appearance or disappearance of HRP 1 year later. On the other hand, in one quarter of patients with persistent HRP, the number of HRP increased or decreased. These results suggest a dynamic nature of HRP detected by CCTA under statin therapy.
References
Stary HC (2000) Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol 20:1177–1178
Virmani R, Burke AP, Farb A, Kolodgie FD (2006) Pathology of the vulnerable plaque. J Am Coll Cardiol 18:C13–C18
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, PROSPECT Investigators et al (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364:226–235
Arbab-Zadeh A, Nakano M, Virmani R, Fuste V (2012) Acute coronary events. Circulation 125:1147–1156
Libby P (2013) Mechanism of acute coronary syndromes and their implacations for therapy. N Engl J Med 368:2004–2013
Kubo T, Maehara A, Mintz GS, Doi H, Tsujita K, Choi SY et al (2010) The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol 55:1590–1597
Zhao Z, Witzenbichler B, Mintz GS, Jaster M, Choi SY, Wu X et al (2013) Dynamic nature of nonculprit coronary artery lesion morphology in STEMI: a serial IVUS analysis from the HORIZONS-AMI trial. JACC Cardiovasc Imaging 6:86–95
Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I et al (2008) Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 359:2324–2336
Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E et al (2008) Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol 52:1724–1732
Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA et al (2008) Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 52:2135–2144
Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T et al (2007) Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 50:319–326
Otsuka K, Fukuda S, Tanaka A, Nakanishi K, Taguchi H, Yoshikawa J et al (2013) Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc Imaging 6:448–457
Thomsen C, Abdulla J (2016) Characteristics of high-risk coronary plaques identified by computed tomographic angiography and associated prognosis: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 17:120–129
Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS et al (1975) A reporting system on patients evaluated for coronary artery disease report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American heart association. Circulation 51(4 Suppl):5–40
Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, ASTEROID Investigators et al (2006) Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295:1556–1565
Wang JC, Normand SL, Mauri L, Kuntz RE (2004) Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation 110:278–284
Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y et al (2015) Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 66:337–346
Fischer C, Hulten E, Belur P, Smith R, Voros S, Villines TC (2013) Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta-analysis. J Cardiovasc Comput Tomogr 7:256–266
Voros S, Rinehart S, Qian Z, Joshi P, Vazquez G, Fischer C et al (2011) Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 4:537–548
Otsuka M, Bruining N, Van Pelt NC, Mollet NR, Ligthart JM, Vourvouri E et al (2008) Quantification of coronary plaque by 64-slice computed tomography: a comparison with quantitative intracoronary ultrasound. Invest Radiol 43:314–321
Brodoefel H, Reimann A, Heuschmid M, Tsiflikas I, Kopp AF, Schroeder S et al (2008) Characterization of coronary atherosclerosis by dual-source computed tomography and HU-based color mapping: a pilot study. Eur Radiol 18:2466–2474
Voros S, Rinehart S, Qian Z, Vazquez G, Anderson H, Murrieta L et al (2011) Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (assessment of tissue characteristics, lesion morphology, and hemodynamics by angiography with fractional flow reserve, intravascular ultrasound and virtual histology, and noninvasive computed tomography in atherosclerotic plaques) I study. JACC Cardiovasc Interv 4:198–208
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Iwasaki, K., Matsumoto, T. Dynamic change of high-risk plaque detected by coronary computed tomographic angiography in patients with subclinical coronary artery disease. Int J Cardiovasc Imaging 32, 1667–1673 (2016). https://doi.org/10.1007/s10554-016-0957-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0957-2