Abstract
Background
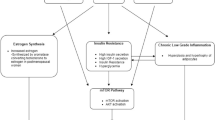
Physical activity has been shown to affect the mammalian target of rapamycin (mTOR) signaling pathway and consequently breast carcinogenesis. Given that Black women in the USA are less physically active, it is not well understood whether there are gene–environment interactions between mTOR pathway genes and physical activity in relation to breast cancer risk in Black women.
Methods
The study included 1398 Black women (567 incident breast cancer cases and 831 controls) from the Women’s Circle of Health Study (WCHS). We examined interactions between 43 candidate single-nucleotide polymorphisms (SNPs) in 20 mTOR pathway genes with levels of vigorous physical activity in relation to breast cancer risk overall and by ER-defined subtypes using Wald test with 2-way interaction term and multivariable logistic regression.
Results
AKT1 rs10138227 (C > T) and AKT1 rs1130214 (C > A) were only associated with a decreased risk of ER + breast cancer among women with vigorous physical activity (odds ratio [OR] = 0.15, 95% confidence interval (CI) 0.04, 0.56, for each copy of the T allele, p-interaction = 0.007 and OR = 0.51, 95% CI 0.27, 0.96, for each copy of the A allele, p-interaction = 0.045, respectively). MTOR rs2295080 (G > T) was only associated with an increased risk of ER + breast cancer among women with vigorous physical activity (OR = 2.24, 95% CI 1.16, 4.34, for each copy of the G allele; p-interaction = 0.043). EIF4E rs141689493 (G > A) was only associated with an increased risk of ER- breast cancer among women with vigorous physical activity (OR = 20.54, 95% CI 2.29, 184.17, for each copy of the A allele; p-interaction = 0.003). These interactions became non-significant after correction for multiple testing (FDR-adjusted p-value > 0.05).
Conclusion
Our findings suggest that mTOR genetic variants may interact with physical activity in relation to breast cancer risk in Black women. Future studies should confirm these findings.
Similar content being viewed by others
Data availability
Data will be made available on request.
References
Hursting SD, Lashinger LM, Wheatley KW, Rogers CJ, Colbert LH, Perkins SN (2008) Reducing the weight of cancer: mechanistic targets for breaking the obesity – carcinogenesis link. Best Pract Res Clin Endocrinol Metab 22(4):659–669. https://doi.org/10.1016/j.beem.2008.08.009
Liu P, Cheng H, Roberts TM, Zhao JJ (2011) Targeting the phosphoinositide 3-kinase (PI3K) pathway in cancer. Nat Rev Drug Discov 8(8):627–644
Altomare DA, Khaled AR (2012) Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr Med Chem 19(22):3748–3762
Ma B, Shan M-H, Sun G, Ren G-H, Dong C, Yao X et al (2015) Immunohistochemical analysis of phosphorylated mammalian target of rapamycin and its downstream signaling components in invasive breast cancer. Mol Med Rep 12:5246–5254
Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG (2005) Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol 25(7):2558–2572
Gingras A, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF et al (1999) Regulation of 4E-BP1 phosphorylation : a novel two-step mechanism. Genes Dev 13(11):1422–1437
Gingras A, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK et al (2001) Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev 15(21):2852–2864
Laplante M, David M (2009) mTOR signaling at a glance. Cell Sci 122:3589–3594
Stephens L, Anderson K, Stokoe D, Erdjument-bromage H, Painter GF, Holmes AB et al (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279(5351):710–714
Manning B, Cantley L (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274
Zarogoulidis P, Lampaki S, Francis Turner J, Huang H, Kakolyris S, Syrigos K et al (2014) mTOR pathway: a current, up-to-date mini-review. Oncol Lett 8(6):2367–2370
De AME, Erhart G, Buck K, Mu E, Hubalek M, Fiegl H et al (2013) Polymorphisms in the gene regions of the adaptor complex LAMTOR2 / LAMTOR3 and their association with breast cancer risk. PLoS ONE 8(1):1–8
Mehta MS, Vazquez A, Kulkarni DA, Kerrigan JE, Atwal G, Metsugi S et al (2013) Polymorphic variants in TSC1 and TSC2 and their association with breast cancer phenotypes. Breast Cancer Res Treat 125(3):1–15
Sirisena ND, Adeyemo A, Kuruppu AI, Samaranayake N, Dissanayake VHW (2018) Genetic variants associated with clinicopathological profiles in sporadic breast cancer in Sri Lankan women. J Breast Cancer 21(2):165–172
Cheng T-YD, Shankar J, Zirpoli G, Roberts MR, Hong C-C, Bandera EV et al (2017) Genetic variants in the mTOR pathway and interaction with body size and weight gain on breast cancer risk in African-American and European-American women. Cancer Causes Control 27(8):965–976
Cheng TD, Ambrosone CB, Hong C, Lunetta KL, Liu S, Hu Q et al (2015) Genetic variants in the mTOR pathway and breast cancer risk in African American women. Carcinogenesis 00(2):1–7
Wang S, Huo D, Ogundiran TO, Ojengbede O, Nathanson KL, Nemesure B et al (2017) Association of breast cancer risk and the mTOR pathway in women of African ancestry in ‘The Root ’ Consortium. Carcinogenesis 38(8):789–796
Campa D, Claus R, Dostal L, Stein A, Karina JC, Benetou V et al (2011) Variation in genes coding for AMP-activated protein kinase (AMPK) and breast cancer risk in the European Prospective Investigation on Cancer (EPIC). Breast Cancer Res Treat 127:761–767
Zhao Y, Diao Y, Wang X, Lin S, Wang M, Kang H (2016) Impacts of the mTOR gene polymorphisms rs2536 and rs2295080 on breast cancer risk in the Chinese population. Oncotarget 7(36):58174–58180
Ren H, Wang X, Lin S (2014) Associations between C1772T polymorphism in hypoxia-inducible factor-1a gene and breast Cancer: a meta-analysis. Med Sci Monit 20:2578–2583
Li X, Zhang R, Liu Z, Li S, Xu H (2017) The genetic variants in the PTEN/PI3K/AKT pathway predict susceptibility and CE(A)F chemotherapy response to breast cancer and clinical outcomes. Oncotarget 8(12):20252–20265
Wang Y, Zhang H, Lin M, Wang Y (2018) Association of FGFR2 and PI3KCA genetic variants with the risk of breast cancer in a Chinese population. Cancer Manag Res 10:1305–1311
Lynch BM, Neilson HKFC (2011) Physical activity and breast cancer prevention. Recent Results Cancer Res 186:13–42
Friedenreich C (2010) The role of physical activity in breast cancer etiology. Semin Oncol 37(3):297–302
Gong Z, Elisa CH, Lucile VB, Troester MA, Kathryn SP, Gary AM et al (2016) Vigorous physical activity and risk of breast cancer in the African American breast cancer epidemiology and risk consortium. Breast Cancer Res Treat 159(2):347–356
Sanderson M, Lipworth L, Shen-miller D, Nechuta S (2015) Energy-related indicators and breast cancer risk among White and Black women. PLoS ONE 10(4):8–9
Rosenberg L, Palmer J, Bethea T, Ban Y, Kipping-Ruane K, Adams-Campbell L (2015) A prospective study of physical activity and breast cancer incidence in African-American women. Cancer Epidemiol Biomarkers Prev 23(11):2522–2531
Sheppard V, Makambi K, Taylor T, Wallington S, Sween J, Adams-Campbell L (2013) Physical activity reduces breast cancer risk in African American women. Ethn Dis 21(4):406–411
Cohen SS, Matthews CE, Bradshaw PT, Lipworth L, Buchowski MS, Signorello LBBW (2014) Sedentary behavior, physical activity, and likelihood of breast cancer among black and white women: a report from the Southern community cohort study. Cancer Prev Res 6(6):566–576
Adams-campbell LL, Rosenberg L, Rao RS, Palmer JR (2001) Strenuous physical activity and breast cancer risk in African-American women. J Natl Med Assoc 93(7):267–275
Bernstein L, Patel AV, Ursin G, Sullivan-halley J, Press F, Deapen D et al (2005) Lifetime recreational exercise activity and breast cancer risk among black women and white women. J Natl Cancer Inst 97(22):1671–1679
John EM, Horn-ross PL, Koo J (2003) Lifetime physical activity and breast cancer risk in a multiethnic population : the San Francisco Bay area breast cancer study. Cancer Epidemiol Biomark Prevent 12(11):1143–1152
Ratnasinghe LD, Modali RV, Seddon MBLT (2010) Physical activity and reduced breast cancer risk: a multinational study. Nutr Cancer 62(4):425–435. https://doi.org/10.1080/01635580903441295
Agostini D, Natalucci V, Baldelli G, De SM, Zeppa SD, Vallorani L et al (2018) New insights into the role of exercise in inhibiting mTOR signaling in triple-negative breast cancer. Oxid Med Cell Longev 2018(9):5896786
Jiang W, Zhu Z, Thompson HJ (2013) Effects of limiting energy availability via diet and physical activity on mammalian target of rapamycin-related signaling in rat mammary carcinomas. Carcinogenesis 34(2):378–387
Center Office of Minority Health Resource. Obesity and African Americans [Internet]. 2020. Available from: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=25
Bandera EV, Chandran U, Hong C-C, Troester MA, Bethea TN, Adams-Campbell LL et al (2015) Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER consortium. Breast Cancer Res Treat 150(3):655–666
World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective [Internet]. 2018. Available from: dietandcancerreport.org
World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and breast cancer [Internet]. 2018. Available from: dietandcancerreport.org
Thompson HJ, Jiang W, Zhu Z, Collins F (2015) Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life 61(9):895–901
Ambrosone CB, Ciupak GL, Bandera EV, Jandorf L, Bovbjerg DH, Zirpoli G et al (2009) Conducting molecular epidemiological research in the age of HIPAA: a multi-institutional case-control study of breast cancer in African-American and European-American women. J Oncol 2009:871250
Bandera EV, Chandran U, Zirpoli G, Mccann SE, Ciupak G, Ambrosone CB (2013) Rethinking sources of representative controls for the conduct of case – control studies in minority populations. BMC Med Res Methodol 13(1):1
Physical Activity Guidelines Advisory Committee (2018) Physical Activity Guidelines Advisory Committee Scientific Report. U.S. Department of Health and Human Services, Washington, DC
Bandera EV, Chandran U, Zirpoli G, Gong Z, Mccann SE, Hong C et al (2013) Body fatness and breast cancer risk in women of African ancestry. BMC Cancer 13(1):1–13
Stolk L, Perry JR, Chasman DI, He C, Mangino M, Sulem P, Barbalic M, Broer L, Byrne EM, Ernst F, Esko T, Franceschini N, Gudbjartsson DF, Hottenga JJ, Kraft P, McArdle PF, Porcu E, Shin SY, Smith AV, van Wingerden S, Zhai G, Zhuang WV, Albrecht E, Alizade LK (2012) Meta-analyses identify 13 novel loci associated with age at menopause and highlights DNA repair and immune pathways. Nat Genet 44(3):260–268
Elks CE, Perry JR, Sulem P, Chasman DI, Franceschini N, He C, Lunetta KL, Visser JA, Byrne EM, Cousminer DL, Gudbjartsson DF, Esko T, Feenstra B, Hottenga JJ, Koller DL, Kutalik Z, Lin P, Mangino M, Marongiu M, McArdle PF, Smith AV, Stolk L, van Wingerden MA (2011) Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet 42(12):1077–1085
Patterson N, Price AL, Reich D (2006) Population structure and eigenanalysis. PLoS Genet 2(12):e190
Ilozumba MN, Yaghjyan L, Datta S et al (2023) mTOR pathway candidate genes and obesity interaction on breast cancer risk in black women from the Women’s circle of health study. Cancer Causes Control. https://doi.org/10.1007/s10552-022-01657-9
Lin J, Wang J, Greisinger AJ, Grossman HB, Michele R, Dinney CP et al (2011) Energy balance, the PI3K-AKT-mTOR pathway genes and the risk of bladder cancer. Cancer Prev Res (Phila) 3(4):505–517
Shu X, Lin J, Wood CG, Tannir NM, Wu X (2013) Energy balance, polymorphisms in the mTOR pathway, and renal cell carcinoma risk. J Natl Cancer Inst 105(6):424–432
Simons CCJM, Schouten LJ, Godschalk RWL, van Schooten FJ, Stoll M, Van Steen K et al (2022) Polymorphisms in the mTOR-PI3K-Akt pathway, energy balance-related exposures and colorectal cancer risk in the Netherlands Cohort Study. BioData Min 15(1):1–20
Broad Institute. HaploReg v3 [Internet]. 2022. Available from: https://pubs.broadinstitute.org/mammals/haploreg/haploreg_v3.php
Rogers CJ, Colbert LH, Greiner JW, Perkins SN, Hursting SD (2008) Physical activity and cancer prevention pathways and targets for intervention. Sport Med 38(4):271–296
Funding
This work was supported by grants from the US National Institutes of Health, the National Cancer Institute (grant number P01 CA151135 J.R.P. and C.B.A, R01CA098663 to J.R.P.; R01 CA100598 to C.B.A. and E.V.B; R01 CA185623, P30 CA016056, P30 CA072720, K07 CA201334, R37 CA248371); and the Breast Cancer Research Foundation (C.B.A., C–CH).
Author information
Authors and Affiliations
Contributions
MNI and TYC contributed to study conception and design. ZG, JRP, SY, CCH, EVB, and CBA contributed to data acquisition. MNI contributed to writing of the initial draft. MNI contributed to data analysis. MNI, TYC, and LY contributed to data interpretation. KLL, TYC, LY, SD, and JZ contributed to the statistical methods. MNI, TYC, LY, SD, JZ, ZG, GZ, SY, and EB revised the paper. Writing—final review and approval were performed by all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The WCHS protocol was approved by the Institutional Review Boards at Roswell Park Cancer Institute, the Rutgers Cancer Institute of New Jersey, Mount Sinai School of Medicine, and participating hospitals in New York. The current study was approved by the University of Florida’s institutional review board.
Consent to participate
Signed informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ilozumba, M.N., Yaghjyan, L., Datta, S. et al. mTOR pathway candidate genes and physical activity interaction on breast cancer risk in black women from the women’s circle of health study. Breast Cancer Res Treat 199, 137–146 (2023). https://doi.org/10.1007/s10549-023-06902-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06902-6