Abstract
Purpose
There is currently no curative treatment for patients diagnosed with triple-negative breast cancer brain metastases (TNBC-BM). CAR T cells hold potential for curative treatment given they retain the cytolytic activity of a T cell combined with the specificity of an antibody. In this proposal we evaluated the potential of EGFR re-directed CAR T cells as a therapeutic treatment against TNBC cells in vitro and in vivo.
Methods
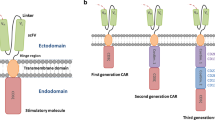
We leveraged a TNBC-BM tissue microarray and a large panel of TNBC cell lines and identified elevated epidermal growth factor receptor (EGFR) expression. Next, we designed a second-generation anti-EGFR CAR T construct incorporating a clinically relevant mAb806 tumor specific single-chain variable fragment (scFv) and intracellular 4-1BB costimulatory domain and CD3\(\zeta\) using a lentivirus system and evaluated in vitro and in vivo anti-tumor activity.
Results
We demonstrate EGFR is enriched in TNBC-BM patient tissue after neurosurgical resection, with six of 13 brain metastases demonstrating both membranous and cytoplasmic EGFR. Eleven of 13 TNBC cell lines have EGFR surface expression ≥ 85% by flow cytometry. EGFR806 CAR T treated mice effectively eradicated TNBC-BM and enhanced mouse survival (log rank p < 0.004).
Conclusion
Our results demonstrates anti-tumor activity of EGFR806 CAR T cells against TNBC cells in vitro and in vivo. Given EGFR806 CAR T cells are currently undergoing clinical trials in primary brain tumor patients without obvious toxicity, our results are immediately actionable against the TNBC-BM patient population.





Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author. All data will be made available to interested parties upon reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA 72:7–33. https://doi.org/10.3322/caac.21708
Brosnan EM, Anders CK (2018) Understanding patterns of brain metastasis in breast cancer and designing rational therapeutic strategies. Ann Transl Med 6:163. https://doi.org/10.21037/atm.2018.04.35
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im S-A, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121. https://doi.org/10.1056/NEJMoa1809615
Garrido-Castro A, Lin N, Polyak K (2019) Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov 9:176–198. https://doi.org/10.1158/2159-8290.Cd-18-1177
Badve SS, Penault-Llorca F, Reis-Filho JS, Deurloo R, Siziopikou KP, D’Arrigo C, Viale G (2021) Determining PD-L1 status in patients with triple-negative breast cancer: lessons learned from IMpassion130. JNCI. https://doi.org/10.1093/jnci/djab121
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G (2014) PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2:361–370. https://doi.org/10.1158/2326-6066.Cir-13-0127
Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP (2008) Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113:2638–2645. https://doi.org/10.1002/cncr.23930
Niikura N, Hayashi N, Masuda N, Takashima S, Nakamura R, Watanabe K, Kanbayashi C, Ishida M, Hozumi Y, Tsuneizumi M, Kondo N, Naito Y, Honda Y, Matsui A, Fujisawa T, Oshitanai R, Yasojima H, Tokuda Y, Saji S, Iwata H (2014) Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat 147:103–112. https://doi.org/10.1007/s10549-014-3090-8
Kim YJ, Kim JS, Kim IA (2018) Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol 144:1803–1816. https://doi.org/10.1007/s00432-018-2697-2
Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, Claus EB, Lee EQ, Wen PY, Haas-Kogan DA, Alexander BM, Lin NU, Aizer AA (2017) Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol 3:1069–1077. https://doi.org/10.1001/jamaoncol.2017.0001
Jaklevic MC (2021) CAR-T therapy is approved for non-hodgkin lymphoma. JAMA 325:1032–1032. https://doi.org/10.1001/jama.2021.3004
Maus MV (2021) CD19 CAR T cells for adults with relapsed or refractory acute lymphoblastic leukaemia. Lancet 398:466–467. https://doi.org/10.1016/s0140-6736(21)01289-7
Akhavan D, Alizadeh D, Wang D, Weist MR, Shepphird JK, Brown CE (2019) CAR T cells for brain tumors: lessons learned and road ahead. Immunol Rev 290:60–84. https://doi.org/10.1111/imr.12773
Choi J, Jung WH, Koo JS (2012) Clinicopathologic features of molecular subtypes of triple negative breast cancer based on immunohistochemical markers. Histol Histopathol 27:1481–1493. https://doi.org/10.14670/hh-27.1481
Liu D, He J, Yuan Z, Wang S, Peng R, Shi Y, Teng X, Qin T (2012) EGFR expression correlates with decreased disease-free survival in triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol 29:401–405. https://doi.org/10.1007/s12032-011-9827-x
Martin V, Botta F, Zanellato E, Molinari F, Crippa S, Mazzucchelli L, Frattini M (2012) Molecular characterization of EGFR and EGFR-downstream pathways in triple negative breast carcinomas with basal like features. Histol Histopathol 27:785–792. https://doi.org/10.14670/hh-27.785
Meseure D, Vacher S, Drak Alsibai K, Trassard M, Susini A, Le Ray C, Lerebours F, Le Scodan R, Spyratos F, Marc Guinebretiere J, Lidereau R, Bieche I (2012) Profiling of EGFR mRNA and protein expression in 471 breast cancers compared with 10 normal tissues: a candidate biomarker to predict EGFR inhibitor effectiveness. Int J Cancer 131:1009–1010. https://doi.org/10.1002/ijc.26434
Park HS, Jang MH, Kim EJ, Kim HJ, Lee HJ, Kim YJ, Kim JH, Kang E, Kim SW, Kim IA, Park SY (2014) High EGFR gene copy number predicts poor outcome in triple-negative breast cancer. Mod Pathol 27:1212–1222. https://doi.org/10.1038/modpathol.2013.251
Viale G, Rotmensz N, Maisonneuve P, Bottiglieri L, Montagna E, Luini A, Veronesi P, Intra M, Torrisi R, Cardillo A, Campagnoli E, Goldhirsch A, Colleoni M (2009) Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat 116:317–328. https://doi.org/10.1007/s10549-008-0206-z
Baselga J, Gómez P, Greil R, Braga S, Climent MA, Wardley AM, Kaufman B, Stemmer SM, Pêgo A, Chan A, Goeminne JC, Graas MP, Kennedy MJ, Ciruelos Gil EM, Schneeweiss A, Zubel A, Groos J, Melezínková H, Awada A (2013) Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol 31:2586–2592. https://doi.org/10.1200/jco.2012.46.2408
Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX, Liu MC, Storniolo AM, Rimawi MF, Forero-Torres A, Wolff AC, Hobday TJ, Ivanova A, Chiu WK, Ferraro M, Burrows E, Bernard PS, Hoadley KA, Perou CM, Winer EP (2012) TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 30:2615–2623. https://doi.org/10.1200/jco.2010.34.5579
Trédan O, Campone M, Jassem J, Vyzula R, Coudert B, Pacilio C, Prausova J, Hardy-Bessard AC, Arance A, Mukhopadhyay P, Aloe A, Roché H (2015) Ixabepilone alone or with cetuximab as first-line treatment for advanced/metastatic triple-negative breast cancer. Clin Breast Cancer 15:8–15. https://doi.org/10.1016/j.clbc.2014.07.007
Fenn K, Maurer M, Lee SM, Crew KD, Trivedi MS, Accordino MK, Hershman DL, Kalinsky K (2020) Phase 1 study of erlotinib and metformin in metastatic triple-negative breast cancer. Clin Breast Cancer 20:80–86. https://doi.org/10.1016/j.clbc.2019.08.004
Symonds L, Linden H, Gadi V, Korde L, Rodler E, Gralow J, Redman M, Baker K, Wu QV, Jenkins I, Kurland B, Garrison M, Smith J, Anderson J, Van Haelst C, Specht J (2019) Combined targeted therapies for first-line treatment of metastatic triple negative breast cancer-A Phase II trial of weekly nab-paclitaxel and bevacizumab followed by maintenance targeted therapy with bevacizumab and erlotinib. Clin Breast Cancer 19:e283–e296. https://doi.org/10.1016/j.clbc.2018.12.008
Costa R, Shah AN, Santa-Maria CA, Cruz MR, Mahalingam D, Carneiro BA, Chae YK, Cristofanilli M, Gradishar WJ, Giles FJ (2017) Targeting Epidermal Growth Factor Receptor in triple negative breast cancer: new discoveries and practical insights for drug development. Cancer Treat Rev 53:111–119. https://doi.org/10.1016/j.ctrv.2016.12.010
Nakai K, Hung MC, Yamaguchi H (2016) A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res 6:1609–1623
Song W, Hwang Y, Youngblood VM, Cook RS, Balko JM, Chen J, Brantley-Sieders DM (2017) Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers. Oncogene 36:5620–5630. https://doi.org/10.1038/onc.2017.170
Priceman SJ, Tilakawardane D, Jeang B, Aguilar B, Murad JP, Park AK, Chang W-C, Ostberg JR, Neman J, Jandial R, Portnow J, Forman SJ, Brown CE (2018) Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2(+) breast cancer metastasis to the brain. Clin Cancer Res 24:95–105. https://doi.org/10.1158/1078-0432.CCR-17-2041
Bryan S, Witzel I, Borgmann K, Oliveira-Ferrer L (2021) Molecular mechanisms associated with brain metastases in HER2-positive and triple negative breast cancers. Cancers (Basel) 13:4137. https://doi.org/10.3390/cancers13164137
Grupka NL, Lear-Kaul KC, Kleinschmidt-DeMasters BK, Singh M (2004) Epidermal growth factor receptor status in breast cancer metastases to the central nervous system. Comparison with HER-2/neu status. Arch Pathol Lab Med 128:974–979. https://doi.org/10.5858/2004-128-974-egfrsi
Xia L, Zheng Z, Liu J-y, Chen Y-j, Ding J, Hu G-s, Hu Y-h, Liu S, Luo W-x, Xia N-s, Liu W (2021) Targeting triple-negative breast cancer with combination therapy of EGFR CAR T cells and CDK7 inhibition. Cancer Immunol Res 9:707–722. https://doi.org/10.1158/2326-6066.Cir-20-0405
Xia L, Zheng ZZ, Liu JY, Chen YJ, Ding JC, Xia NS, Luo WX, Liu W (2020) EGFR-targeted CAR-T cells are potent and specific in suppressing triple-negative breast cancer both in vitro and in vivo. Clin Transl Immunol 9:e01135. https://doi.org/10.1002/cti2.1135
Hübner J, Raschke M, Rütschle I, Gräßle S, Hasenberg T, Schirrmann K, Lorenz A, Schnurre S, Lauster R, Maschmeyer I, Steger-Hartmann T, Marx U (2018) Simultaneous evaluation of anti-EGFR-induced tumour and adverse skin effects in a microfluidic human 3D co-culture model. Sci Rep 8:15010. https://doi.org/10.1038/s41598-018-33462-3
Laux I, Jain A, Singh S, Agus DB (2006) Epidermal growth factor receptor dimerization status determines skin toxicity to HER-kinase targeted therapies. Br J Cancer 94:85–92. https://doi.org/10.1038/sj.bjc.6602875
Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, Bailey SR, Boroughs AC, Frigault MJ, Leick MB, Scarfò I, Cetrulo CL, Demehri S, Nahed BV, Cahill DP, Wakimoto H, Curry WT, Carter BS, Maus MV (2019) CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol 37:1049–1058. https://doi.org/10.1038/s41587-019-0192-1
Jungbluth AA, Stockert E, Huang HJ, Collins VP, Coplan K, Iversen K, Kolb D, Johns TJ, Scott AM, Gullick WJ, Ritter G, Cohen L, Scanlan MJ, Cavenee WK, Old LJ (2003) A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci USA 100:639–644. https://doi.org/10.1073/pnas.232686499
Luwor RB, Johns TG, Murone C, Huang HJ, Cavenee WK, Ritter G, Old LJ, Burgess AW, Scott AM (2001) Monoclonal antibody 806 inhibits the growth of tumor xenografts expressing either the de2-7 or amplified epidermal growth factor receptor (EGFR) but not wild-type EGFR. Cancer Res 61:5355–5361
Panousis C, Rayzman VM, Johns TG, Renner C, Liu Z, Cartwright G, Lee FT, Wang D, Gan H, Cao D, Kypridis A, Smyth FE, Brechbiel MW, Burgess AW, Old LJ, Scott AM (2005) Engineering and characterisation of chimeric monoclonal antibody 806 (ch806) for targeted immunotherapy of tumours expressing de2-7 EGFR or amplified EGFR. Br J Cancer 92:1069–1077. https://doi.org/10.1038/sj.bjc.6602470
Simon N, Antignani A, Sarnovsky R, Hewitt SM, FitzGerald D (2016) Targeting a cancer-specific epitope of the epidermal growth factor receptor in triple-negative breast cancer. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djw028
Scott AM, Lee FT, Tebbutt N, Herbertson R, Gill SS, Liu Z, Skrinos E, Murone C, Saunder TH, Chappell B, Papenfuss AT, Poon AM, Hopkins W, Smyth FE, MacGregor D, Cher LM, Jungbluth AA, Ritter G, Brechbiel MW, Murphy R, Burgess AW, Hoffman EW, Johns TG, Old LJ (2007) A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci USA 104:4071–4076. https://doi.org/10.1073/pnas.0611693104
Vitanza N, Gust J, Wilson A, Huang W, Perez F, Wright J, Leary S, Cole B, Albert C, Pinto N, Orentas R, Jensen M, Park J (2020) IMMU-03. UPDATES ON BRAINCHILD-01, -02, AND -03: PHASE 1 LOCOREGIONAL CAR T CELL TRIALS TARGETING HER2, EGFR, AND B7–H3 FOR CHILDREN WITH RECURRENT CNS TUMORS AND DIPG. Neuro-Oncology 22:iii360–iii360. https://doi.org/10.1093/neuonc/noaa222.360
Atlas P (2022) In:
Huerta JJ, Diaz-Trelles R, Naves FJ, Llamosas MM, Del Valle ME, Vega JA (1996) Epidermal growth factor receptor in adult human dorsal root ganglia. Anat Embryol (Berl) 194:253–257. https://doi.org/10.1007/bf00187136
Kornblum HI, Gall CM, Seroogy KB, Lauterborn JC (1995) A subpopulation of striatal gabaergic neurons expresses the epidermal growth factor receptor. Neuroscience 69:1025–1029. https://doi.org/10.1016/0306-4522(95)00392-v
Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, Chang WC, Bretzlaff W, Starr R, Priceman S, Ostberg JR, Forman SJ, Brown CE (2015) Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther 23:757–768. https://doi.org/10.1038/mt.2014.208
Ravanpay AC, Gust J, Johnson AJ, Rolczynski LS, Cecchini M, Chang CA, Hoglund VJ, Mukherjee R, Vitanza NA, Orentas RJ, Jensen MC (2019) EGFR806-CAR T cells selectively target a tumor-restricted EGFR epitope in glioblastoma. Oncotarget 10:7080–7095. https://doi.org/10.18632/oncotarget.27389
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121:2750–2767. https://doi.org/10.1172/JCI45014
Agarwal S, Hanauer JDS, Frank AM, Riechert V, Thalheimer FB, Buchholz CJ (2020) In vivo generation of CAR T cells selectively in human CD4(+) lymphocytes. Mol Ther 28:1783–1794. https://doi.org/10.1016/j.ymthe.2020.05.005
Wang D, Aguilar B, Starr R, Alizadeh D, Brito A, Sarkissian A, Ostberg JR, Forman SJ, Brown CE (2018) Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight. https://doi.org/10.1172/jci.insight.99048
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D’Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B (2016) Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 375:2561–2569. https://doi.org/10.1056/NEJMoa1610497
O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, Isaacs R, Mohan S, Plesa G, Lacey SF, Navenot JM, Zheng Z, Levine BL, Okada H, June CH, Brogdon JL, Maus MV (2017) A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaa0984
Sirkisoon SR, Carpenter RL, Rimkus T, Miller L, Metheny-Barlow L, Lo H-W (2016) EGFR and HER2 signaling in breast cancer brain metastasis. Front Biosci (Elite Ed) 8:245–263. https://doi.org/10.2741/E765
Heczey A (2019) Alliance of the titans: an effective combination of a TKI with CAR T cells. Mol Ther 27:1348–1349. https://doi.org/10.1016/j.ymthe.2019.07.008
Simon N, Antignani A, Sarnovsky R, Hewitt SM, FitzGerald D (2016) Targeting a cancer-specific epitope of the epidermal growth factor receptor in triple-negative breast cancer. JNCI. https://doi.org/10.1093/jnci/djw028
Guo P, Pu T, Chen S, Qiu Y, Zhong X, Zheng H, Chen L, Bu H, Ye F (2017) Breast cancers with EGFR and HER2 co-amplification favor distant metastasis and poor clinical outcome. Oncol Lett 14:6562–6570. https://doi.org/10.3892/ol.2017.7051
Choe JH, Watchmaker PB, Simic MS, Gilbert RD, Li AW, Krasnow NA, Downey KM, Yu W, Carrera DA, Celli A, Cho J, Briones JD, Duecker JM, Goretsky YE, Dannenfelser R, Cardarelli L, Troyanskaya O, Sidhu SS, Roybal KT, Okada H, Lim WA (2021) SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med. https://doi.org/10.1126/scitranslmed.abe7378
Dees S, Ganesan R, Singh S, Grewal IS (2020) Emerging CAR-T cell therapy for the treatment of triple-negative breast cancer. Mol Cancer Ther 19:2409–2421. https://doi.org/10.1158/1535-7163.Mct-20-0385
Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR (2012) Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood 119:72–82. https://doi.org/10.1182/blood-2011-07-366419
Wang X, Naranjo A, Brown CE, Bautista C, Wong CW, Chang WC, Aguilar B, Ostberg JR, Riddell SR, Forman SJ, Jensen MC (2012) Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. J Immunother 35:689–701. https://doi.org/10.1097/CJI.0b013e318270dec7
Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, Gao P, Bandyopadhyay S, Sun H, Zhao Z, Lundh S, Pruteanu-Malinici I, Nobles CL, Maji S, Frey NV, Gill SI, Tian L, Kulikovskaya I, Gupta M, Ambrose DE, Davis MM, Fraietta JA, Brogdon JL, Young RM, Chew A, Levine BL, Siegel DL, Alanio C, Wherry EJ, Bushman FD, Lacey SF, Tan K, June CH (2022) Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature 602:503–509. https://doi.org/10.1038/s41586-021-04390-6
Xhangolli I, Dura B, Lee G, Kim D, Xiao Y, Fan R (2019) Single-cell analysis of CAR-T cell activation reveals A mixed TH1/TH2 response independent of differentiation. Genom Proteom Bioinform 17:129–139. https://doi.org/10.1016/j.gpb.2019.03.002
Yang Y, Kohler ME, Chien CD, Sauter CT, Jacoby E, Yan C, Hu Y, Wanhainen K, Qin H, Fry TJ (2017) TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aag1209
Liadi I, Singh H, Romain G, Rey-Villamizar N, Merouane A, Adolacion JR, Kebriaei P, Huls H, Qiu P, Roysam B, Cooper LJ, Varadarajan N (2015) Individual motile CD4(+) T cells can participate in efficient multikilling through conjugation to multiple tumor cells. Cancer Immunol Res 3:473–482. https://doi.org/10.1158/2326-6066.Cir-14-0195
Tsang JE, Urner LM, Kim G, Chow K, Baufeld L, Faull K, Cloughesy TF, Clark PM, Jung ME, Nathanson DA (2020) Development of a potent brain-penetrant EGFR tyrosine kinase inhibitor against malignant brain tumors. ACS Med Chem Lett 11:1799–1809. https://doi.org/10.1021/acsmedchemlett.9b00599
Funding
This work was funded by the University of Kansas Cancer Center.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DA, SS, SS, JJ, and CW, BS, and JZ. The first draft of the manuscript was written by DA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki and its later amendments or comparable ethical standards. Ethical approval of this study was granted by the University of Kansas Medical Center Institutional Review Board (IRB) and IACUC on September 21, 2021.
Informed consent
Informed consent was obtained from all University of Kansas Medical Center Biorepository Core Facility for all patient tissue presented in this study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for de-identified immunohistochemistry in Fig. 1A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Subham, S., Jeppson, J.D., Worcester, C. et al. EGFR as a potent CAR T target in triple negative breast cancer brain metastases. Breast Cancer Res Treat 197, 57–69 (2023). https://doi.org/10.1007/s10549-022-06783-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06783-1