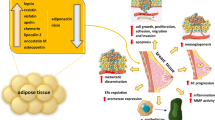
Abstract
Purpose
Limited epidemiologic data are available on the expression of adipokines leptin (LEP) and adiponectin (ADIPOQ) and adipokine receptors (LEPR, ADIPOR1, ADIPOR2) in the breast tumor microenvironment (TME). The associations of gene expression of these biomarkers with tumor clinicopathology are not well understood.
Methods
NanoString multiplexed assays were used to assess the gene expression levels of LEP, LEPR, ADIPOQ, ADIPOR1, and ADIPOR2 within tumor tissues among 162 Black and 55 White women with newly diagnosed breast cancer. Multivariate mixed effects models were used to estimate associations of gene expression with breast tumor clinicopathology (overall and separately among Blacks).
Results
Black race was associated with lower gene expression of LEPR (P = 0.002) and ADIPOR1 (P = 0.01). Lower LEP, LEPR, and ADIPOQ gene expression were associated with higher tumor grade (P = 0.0007, P < 0.0001, and P < 0.0001, respectively) and larger tumor size (P < 0.0001, P = 0.0005, and P < 0.0001, respectively). Lower ADIPOQ expression was associated with ER- status (P = 0.0005), and HER2-enriched (HER2-E; P = 0.0003) and triple-negative (TN; P = 0.002) subtypes. Lower ADIPOR2 expression was associated with Ki67+ status (P = 0.0002), ER- status (P < 0.0001), PR- status (P < 0.0001), and TN subtype (P = 0.0002). Associations of lower adipokine and adipokine receptor gene expression with ER-, HER2-E, and TN subtypes were confirmed using data from The Cancer Genome Atlas (P-values < 0.005).
Conclusion
These findings suggest that lower expression of ADIPOQ, ADIPOR2, LEP, and LEPR in the breast TME might be indicators of more aggressive breast cancer phenotypes. Validation of these findings are warranted to elucidate the role of the adipokines and adipokine receptors in long-term breast cancer prognosis.



Similar content being viewed by others
Abbreviations
- ADIPOQ:
-
Adiponectin
- ADIPOR1:
-
Adiponectin receptor 1
- ADIPOR2:
-
Adiponectin receptor 2
- ANOVA:
-
Analysis of variance
- BC:
-
Breast cancer
- BMI:
-
Body mass index
- DBBR:
-
Data Bank and Biorepository
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- HER2-E:
-
Non-luminal HER2-enriched subtype
- IGF-1:
-
Insulin-like growth factor 1
- IHC:
-
Immunohistochemistry
- IRS1:
-
Insulin receptor substrate 1
- JAK/STAT:
-
Janus kinase-signal transducer and activator of transcription
- LEP:
-
Leptin
- LEPR:
-
Leptin receptor
- MAPK:
-
Mitogen-activated protein kinase
- PR:
-
Progesterone receptor
- SD:
-
Standard deviation
- SOCS3:
-
Suppressor of cytokine signaling 3
- TCGA:
-
The Cancer Genome Atlas
- TN:
-
Triple-negative subtype
- TNF-α:
-
Tumor necrosis factor alpha
- WCHS:
-
Women’s Circle of Health Study
References
Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose most abundant gene transcript 1). Biochem Biophys Res Commun 221(2):286–289
Haluzik M, Parizkova J, Haluzik MM (2004) Adiponectin and its role in the obesity-induced insulin resistance and related complications. Physiol Res 53(2):123–129
Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Perrone D, Penault-Llorca F, Guillot J, Vasson MP (2008) Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology 53(4):484–487
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6):2548–2556
Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R (2006) Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 345(1):271–279
Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, Pecquery R (2008) Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep 20(4):971–977
Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A (2005) Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem 280(18):18341–18347
Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R (2001) Tumor necrosis factor alpha is a negative regulator of resistin gene expression and secretion in 3T3-L1 adipocytes. Biochem Biophys Res Commun 288(4):1027–1031
Makimura H, Mizuno TM, Bergen H, Mobbs CV (2002) Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. Am J Physiol Endocrinol Metab 283(6):E1266-1271
Hu X, Juneja SC, Maihle NJ, Cleary MP (2002) Leptin–a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst 94(22):1704–1711
O’Brien SN, Welter BH, Price TM (1999) Presence of leptin in breast cell lines and breast tumors. Biochem Biophys Res Commun 259(3):695–698
Foryst-Ludwig A, Kreissl MC, Benz V, Brix S, Smeir E, Ban Z, Januszewicz E, Salatzki J, Grune J, Schwanstecher AK et al (2015) Adipose tissue lipolysis promotes exercise-induced cardiac hypertrophy involving the lipokine C16:1n7-palmitoleate. J Biol Chem 290(39):23603–23615
Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y (2002) Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun 293(1):622–628
Cao R, Brakenhielm E, Wahlestedt C, Thyberg J, Cao Y (2001) Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci USA 98(11):6390–6395
Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS (2001) Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med 33(2):95–102
Laud K, Gourdou I, Pessemesse L, Peyrat JP, Djiane J (2002) Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol Cell Endocrinol 188(1–2):219–226
Schmidt S, Monk JM, Robinson LE, Mourtzakis M (2015) The integrative role of leptin, oestrogen and the insulin family in obesity-associated breast cancer: potential effects of exercise. Obes Rev 16(6):473–487
Mocino-Rodriguez MD, Santillan-Benitez JG, Dozal-Dominguez DS, Hernandez-Navarro MD, Flores-Merino MV, Sandoval-Cabrera A, Garcia Vazquez FJ (2017) Expression of AdipoR1 and AdipoR2 receptors as leptin-breast cancer regulation mechanisms. Dis Markers 2017:4862016
Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F (2011) Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer 47(1):33–43
Llanos AAM, Lin Y, Chen W, Yao S, Norin J, Chekmareva MA, Omene C, Cong L, Omilian AR, Khoury T et al (2020) Immunohistochemical analysis of adipokine and adipokine receptor expression in the breast tumor microenvironment: associations of lower leptin receptor expression with estrogen receptor-negative status and triple-negative subtype. Breast Cancer Res 22(1):18
Ambrosone CB, Zirpoli G, Ruszczyk M, Shankar J, Hong CC, McIlwain D, Roberts M, Yao S, McCann SE, Ciupak G et al (2014) Parity and breastfeeding among African–American women: differential effects on breast cancer risk by estrogen receptor status in the Women’s Circle of Health Study. Cancer Causes Control 25(2):259–265
Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70
Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, Trevino V, Shen H, Laird PW, Levine DA et al (2013) Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 4:2612
Mauro L, Pellegrino M, De Amicis F, Ricchio E, Giordano F, Rizza P, Catalano S, Bonofiglio D, Sisci D, Panno ML et al (2014) Evidences that estrogen receptor alpha interferes with adiponectin effects on breast cancer cell growth. Cell Cycle 13(4):553–564
Mauro L, Pellegrino M, Giordano F, Ricchio E, Rizza P, De Amicis F, Catalano S, Bonofiglio D, Panno ML, Ando S (2015) Estrogen receptor-alpha drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J 29(5):2150–2160
Yoon YS, Kwon AR, Lee YK, Oh SW (2019) Circulating adipokines and risk of obesity related cancers: a systematic review and meta-analysis. Obes Res Clin Pract 13:329–339
Revillion F, Charlier M, Lhotellier V, Hornez L, Giard S, Baranzelli MC, Djiane J, Peyrat JP (2006) Messenger RNA expression of leptin and leptin receptors and their prognostic value in 322 human primary breast cancers. Clin Cancer Res 12(7 Pt 1):2088–2094
Miyoshi Y, Funahashi T, Tanaka S, Taguchi T, Tamaki Y, Shimomura I, Noguchi S (2006) High expression of leptin receptor mRNA in breast cancer tissue predicts poor prognosis for patients with high, but not low, serum leptin levels. Int J Cancer 118(6):1414–1419
Pfeiler G, Treeck O, Wenzel G, Goerse R, Hartmann A, Schmitz G, Ortmann O (2009) Influence of insulin resistance on adiponectin receptor expression in breast cancer. Maturitas 63(3):253–256
Sultana R, Kataki AC, Borthakur BB, Basumatary TK, Bose S (2017) Imbalance in leptin-adiponectin levels and leptin receptor expression as chief contributors to triple negative breast cancer progression in Northeast India. Gene 621:51–58
Goovaerts T, Steyaert S, Vandenbussche CA, Galle J, Thas O, Van Criekinge W, De Meyer T (2018) A comprehensive overview of genomic imprinting in breast and its deregulation in cancer. Nat Commun 9(1):4120
Tahiri A, Aure MR, Kristensen VN (2018) MicroRNA networks in breast cancer cells. Methods Mol Biol 1711:55–81
Koedoot E, Wolters L, van de Water B, Devedec SEL (2019) Splicing regulatory factors in breast cancer hallmarks and disease progression. Oncotarget 10(57):6021–6037
Mendoza-Perez J, Gu J, Herrera LA, Tannir NM, Zhang S, Matin S, Karam JA, Wood CG, Wu X (2017) Prognostic significance of promoter CpG island methylation of obesity-related genes in patients with nonmetastatic renal cell carcinoma. Cancer 123(18):3617–3627
Masuda N, Ohnishi T, Kawamoto S, Monden M, Okubo K (1999) Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res 27(22):4436–4443
Reis PP, Waldron L, Goswami RS, Xu W, Xuan Y, Perez-Ordonez B, Gullane P, Irish J, Jurisica I, Kamel-Reid S (2011) mRNA transcript quantification in archival samples using multiplexed, color-coded probes. BMC Biotechnol 11:46
Acknowledgements
We are sincerely appreciative of the breast cancer advocates, community partners, and all of the women who generously donated their time to participate in the study. We are equally grateful to the highly motivated, hardworking research personnel of the Women’s Circle of Health Study at the Rutgers School of Public Health, Rutgers Cancer Institute of New Jersey, Roswell Park Comprehensive Cancer Center, Mount Sinai School of Medicine (now Icahn School of Medicine at Mount Sinai), and the New Jersey State Cancer Registry.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: P01CA151135 (awarded to C.B. Ambrosone), P30CA072720 (awarded to S. Libutti), R01CA100598 (awarded to C.B. Ambrosone), R01CA185623 (awarded to E.V. Bandera, K. Demissie, and C.C. Hong), K01CA193527 (awarded to A.A.M. Llanos), and K08CA172722 (awarded to C. Omene). Research in this publication was also supported by the U.S. Army Medical Research and Development Command under award number DAMD‐17‐01‐1‐0334 (awarded to D.H. Bovbjerg), the Breast Cancer Research Foundation (awarded to C.B. Ambrosone and C.C. Hong), and a gift from the Philip L. Hubbell Family (awarded to K. Demissie). Tumor samples were received, processed and tracked under the auspices of the Roswell Park Comprehensive Cancer Center Data Bank and BioRepository Shared Resource, with funding from NCI-CCSG P30CA16056. Services, results and/or products in support of this research project were generated using the Rutgers Cancer Institute of New Jersey Biomedical Informatics Shared Resource (P30CA072720-5917) and the Biospecimen Repository and Histopathology Service Shared Resource (P30CA072720-5919). The New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey Department of Health, is funded by the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute under contract HHSN261201300021I and control No. N01-PC-2013-00021, the National Program of Cancer Registries (NPCR), Centers for Disease Control and Prevention under grant NU5U58DP006279-02-00 as well as the State of New Jersey and the Rutgers Cancer Institute of New Jersey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Institutional Review Boards of all participating institutions. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study (prior to enrollment in the study).
Research involving human and animal participants
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Llanos, A.A.M., Yao, S., Singh, A. et al. Gene expression of adipokines and adipokine receptors in the tumor microenvironment: associations of lower expression with more aggressive breast tumor features. Breast Cancer Res Treat 185, 785–798 (2021). https://doi.org/10.1007/s10549-020-05972-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05972-0