Abstract
Purpose
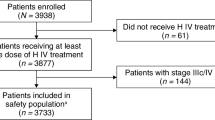
Little data exist for comparing cardiac safety and survival outcomes of trastuzumab/pertuzumab or ado-T emtansine (TDM1) in metastatic breast cancer (MBC) patients enrolled in randomized clinical trial (RCT) vs the real-world.
Methods
This was a retrospective population-based cohort of all patients with MBC treated with trastuzumab/pertuzumab or TDM1 (2012–2017) in Ontario, Canada. Outcomes were incident heart failure (HF) and overall survival (OS). RCT data were obtained from digitizing survival curves and compared with cohort data using Kaplan–Meier analysis. Age-based comparison of outcomes was conducted for patients ≥ 65 years old vs younger than 65.
Results
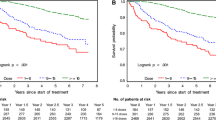
The two cohorts composed of 833 and 397 patients treated with trastuzumab/pertuzumab and TDM1, of whom 5.5% and 7.6% had baseline HF, respectively. Incident HF following trastuzumab/pertuzumab or TDM1 was low (trastuzumab/pertuzumab 1.8 events/100 person years; TDM1 0.02 events/100 person years). The median OS was 39.2 and 56.4 months in the trastuzumab/pertuzumab population-based cohort and CLEOPATRA, respectively. The median OS was 15.4 and 30.9 months in the TDM1 population-based cohort and EMILIA, respectively. Cohort OS was significantly worse than RCT OS (trastuzumab/pertuzumab HR 1.67, 95% CI 1.37–2.03, p < 0.0001; TDM1 HR 2.80, 95% CI 2.27–3.44, p < 0.0001). Older patients had worse OS than younger patients for trastuzumab/pertuzumab (HR 1.60, 95% CI 1.19–2.16, p = 0.0018), but not for TDM1 (HR 1.16, 95% CI 0.81–1.66, p = 0.43).
Conclusion
HF incidence during trastuzumab/pertuzumab or TDM1 therapy in this real-world cohort was low. Survival in this cohort was worse compared to RCT, suggesting that recruitment of patients similar to the real-world population is required.



Similar content being viewed by others
References
Early Breast Cancer Trialists' Collaborative G (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. https://doi.org/10.1016/S0140-6736(05)66544-0
Chia SK, Speers CH, D'Yachkova Y, Kang A, Malfair-Taylor S, Barnett J, Coldman A, Gelmon KA, O'Reilly SE, Olivotto IA (2007) The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 110(5):973–979. https://doi.org/10.1002/cncr.22867
Dafni U, Grimani I, Xyrafas A, Eleftheraki AG, Fountzilas G (2010) Fifteen-year trends in metastatic breast cancer survival in Greece. Breast Cancer Res Treat 119(3):621–631. https://doi.org/10.1007/s10549-009-0630-8
Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P (2005) Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer 104(8):1742–1750. https://doi.org/10.1002/cncr.21359
Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H (2010) Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 28(10):1684–1691. https://doi.org/10.1200/JCO.2009.24.9284
Haque R, Ahmed SA, Inzhakova G, Shi J, Avila C, Polikoff J, Bernstein L, Enger SM, Press MF (2012) Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomark Prev 21(10):1848–1855. https://doi.org/10.1158/1055-9965.EPI-12-0474
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM, Group CS (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119. https://doi.org/10.1056/NEJMoa1113216
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K, Group ES (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367(19):1783–1791. https://doi.org/10.1056/NEJMoa1209124
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792. https://doi.org/10.1056/NEJM200103153441101
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684. https://doi.org/10.1056/NEJMoa052122
Guyot P, Ades AE, Ouwens MJ, Welton NJ (2012) Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 12:9. https://doi.org/10.1186/1471-2288-12-9
Gobbini E, Ezzalfani M, Dieras V, Bachelot T, Brain E, Debled M, Jacot W, Mouret-Reynier MA, Goncalves A, Dalenc F, Patsouris A, Ferrero JM, Levy C, Lorgis V, Vanlemmens L, Lefeuvre-Plesse C, Mathoulin-Pelissier S, Petit T, Uwer L, Jouannaud C, Leheurteur M, Lacroix-Triki M, Cleaud AL, Robain M, Courtinard C, Cailliot C, Perol D, Delaloge S (2018) Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer 96:17–24. https://doi.org/10.1016/j.ejca.2018.03.015
(2010). In: Nass SJ, Moses HL, Mendelsohn J (eds) A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington (DC)
Unger JM, Cook E, Tai E, Bleyer A (2016) The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book 35:185–198. https://doi.org/10.1200/EDBK_156686
Lamont EB, Landrum MB, Keating NL, Archer L, Lan L, Strauss GM, Lilenbaum R, Niell HB, Maurer LH, Kosty MP, Miller AA, Clamon GH, Elias AD, McClay EF, Vokes EE, McNeil BJ (2010) Differences in clinical trial patient attributes and outcomes according to enrollment setting. J Clin Oncol 28(2):215–221. https://doi.org/10.1200/JCO.2008.21.3652
Meyer RM (2010) Generalizing the results of cancer clinical trials. J Clin Oncol 28(2):187–189. https://doi.org/10.1200/JCO.2009.25.8608
Srikanthan A, Amir E (2015) Efficacy-effectiveness gap as an obstacle to translating clinical trials to clinical practice. Eur J Cancer 51(8):905–906. https://doi.org/10.1016/j.ejca.2015.03.017
Le Saux O, Falandry C, Gan HK, You B, Freyer G, Peron J (2016) Inclusion of elderly patients in oncology clinical trials. Ann Oncol 27(9):1799–1804. https://doi.org/10.1093/annonc/mdw259
Miles D, Baselga J, Amadori D, Sunpaweravong P, Semiglazov V, Knott A, Clark E, Ross G, Swain SM (2013) Treatment of older patients with HER2-positive metastatic breast cancer with pertuzumab, trastuzumab, and docetaxel: subgroup analyses from a randomized, double-blind, placebo-controlled phase III trial (CLEOPATRA). Breast Cancer Res Treat 142(1):89–99. https://doi.org/10.1007/s10549-013-2710-z
Strulov Shachar S, Hurria A, Muss HB (2016) Targeted therapies in older adults with breast cancer: what do we know? J Clin Oncol 34(28):3486–3488. https://doi.org/10.1200/JCO.2016.68.8242
Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, Reed M, Ciatto S, Voogd AC, Brain E, Cutuli B, Terret C, Gosney M, Aapro M, Audisio R (2012) Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol 13(4):e148–160. https://doi.org/10.1016/S1470-2045(11)70383-7
Wildiers H, Kunkler I, Biganzoli L, Fracheboud J, Vlastos G, Bernard-Marty C, Hurria A, Extermann M, Girre V, Brain E, Audisio RA, Bartelink H, Barton M, Giordano SH, Muss H, Aapro M (2007) Management of breast cancer in elderly individuals: recommendations of the International Society of Geriatric Oncology. Lancet Oncol 8(12):1101–1115. https://doi.org/10.1016/S1470-2045(07)70378-9
Donald Harvey WSR, Gwynn I, Sean K, Li C, Miller RS, Monika J, Edward S, Brigham H, Uldrick TS, Komatsoulis GA, Jeremy R, Garrett-Mayer E, Schilsky RL, Schenkel C, Kim ES, Bruinooge SS (2019) Impact of broadening clinical trial eligibility criteria for advanced non-small cell lung cancer patients: Real-world analysis. J Clin Oncol. https://doi.org/10.1200/JCO.2019.37.18_suppl.LBA108
Grimshaw JM, Russell IT (1993) Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet 342(8883):1317–1322. https://doi.org/10.1016/0140-6736(93)92244-n
Braunholtz DA, Edwards SJ, Lilford RJ (2001) Are randomized clinical trials good for us (in the short term)? Evidence for a "trial effect". J Clin Epidemiol 54(3):217–224. https://doi.org/10.1016/s0895-4356(00)00305-x
Unger JM, Barlow WE, Martin DP, Ramsey SD, Leblanc M, Etzioni R, Hershman DL (2014) Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju002
Elkin EB, Hurria A, Mitra N, Schrag D, Panageas KS (2006) Adjuvant chemotherapy and survival in older women with hormone receptor-negative breast cancer: assessing outcome in a population-based, observational cohort. J Clin Oncol 24(18):2757–2764. https://doi.org/10.1200/JCO.2005.03.6053
Meyerhardt JA, Li L, Sanoff HK, Wt C, Schrag D (2012) Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. J Clin Oncol 30(6):608–615. https://doi.org/10.1200/JCO.2011.38.9650
Lakdawalla DN, Shafrin J, Hou N, Peneva D, Vine S, Park J, Zhang J, Brookmeyer R, Figlin RA (2017) Predicting real-world effectiveness of cancer therapies using overall survival and progression-free survival from clinical trials: empirical evidence for the ASCO value framework. Value Health 20(7):866–875. https://doi.org/10.1016/j.jval.2017.04.003
Piccart M PM, Fumagalli D, de Azambuja E, Emma Clark, Michael S. Ewer, Eleonora Restuccia, Guy Jerusalem, Susan Dent, Linda Reaby, Hervé Bonnefoi, Ian Krop, Tsang-Wu Liu, Tadeusz Pieńkowski, Masakazu Toi, Nicolas Wilcken, Michael Andersson, Young-Hyuck Im, Ling-Ming Tseng, Hans-Joachim Lueck, Marco Colleoni, Estefania Monturus, Mihaela Sicoe, Sébastien Guillaume, José Bines, Richard Gelber, Giuseppe Viale, Christoph Thomssen. (2020) Interim overall survival analysis of APHINITY (BIG 4-11): A randomized multicenter, double-blind, placebo-controlled trial comparing chemotherapy plus trastuzumab plus pertuzumab versus chemotherapy plus trastuzumab plus placebo as adjuvant therapy in patients with operable HER2-positive early breast cancer [abstract]. Cancer Res 80(4 Suppl):Abstract nr GS1-04.
Administration FaD US Food and Drug Administration (FDA)-approved prescribing information for trastuzumab emtansine. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf?et_cid=31141095&et_rid=463638624&linkid=http%3a%2f%2fwww.accessdata.fda.gov%2fdrugsatfda_docs%2flabel%2f2013%2f125427lbl.pdf. Accessed 20 Sept 2019
Administration FaD US Food and Drug Administration (FDA)-approved prescribing information for pertuzumab. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125409s113s118lbl.pdf. Accessed 20 Sept 2019
Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, Habel LA, Yood MU, McCarty C, Magid DJ, Wagner EH, Pharmacovigilance Study T (2012) Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 104(17):1293–1305. https://doi.org/10.1093/jnci/djs317
Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP (2012) Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol 60(24):2504–2512. https://doi.org/10.1016/j.jacc.2012.07.068
Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, Smith BD, Hortobagyi GN, Giordano SH (2013) Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol 31(33):4222–4228. https://doi.org/10.1200/JCO.2013.48.7884
McArthur HL, Chia S (2007) Cardiotoxicity of trastuzumab in clinical practice. New Engl J Med 357(1):94–95. https://doi.org/10.1056/NEJMc070065
Tarantini L, Cioffi G, Gori S, Tuccia F, Boccardi L, Bovelli D, Lestuzzi C, Maurea N, Oliva S, Russo G, Faggiano P, Italian Cardio-Oncologic N (2012) Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J Cardiac Fail 18(2):113–119. https://doi.org/10.1016/j.cardfail.2011.10.015
Tarantini L, Gori S, Faggiano P, Pulignano G, Simoncini E, Tuccia F, Ceccherini R, Bovelli D, Lestuzzi C, Cioffi G, Network I (2012) Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: a multicenter cohort analysis. Ann Oncol 23(12):3058–3063. https://doi.org/10.1093/annonc/mds127
Du XL, Xia R, Burau K, Liu CC (2011) Cardiac risk associated with the receipt of anthracycline and trastuzumab in a large nationwide cohort of older women with breast cancer, 1998–2005. Med Oncol 28(Suppl 1):S80–90. https://doi.org/10.1007/s12032-010-9717-7
Gong IY, Verma S, Yan AT, Ko DT, Earle CC, Tomlinson GA, Trudeau ME, Krahn MD, Krzyzanowska MK, Brezden-Masley CB, Gavura S, Peacock S, Chan KK (2016) Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: a population-based study. Breast Cancer Res Treat 157(3):535–544. https://doi.org/10.1007/s10549-016-3823-y
Swain SM, Ewer MS, Cortes J, Amadori D, Miles D, Knott A, Clark E, Benyunes MC, Ross G, Baselga J (2013) Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist 18(3):257–264. https://doi.org/10.1634/theoncologist.2012-0448
Yu AF, Manrique C, Pun S, Liu JE, Mara E, Fleisher M, Patil S, Jones LW, Steingart RM, Hudis CA, Dang CT (2016) Cardiac safety of paclitaxel plus trastuzumab and pertuzumab in patients with HER2-positive metastatic breast cancer. Oncologist 21(4):418–424. https://doi.org/10.1634/theoncologist.2015-0321
Guglin M, Krischer J, Tamura R, Fink A, Bello-Matricaria L, McCaskill-Stevens W, Munster PN (2019) Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol 73(22):2859–2868. https://doi.org/10.1016/j.jacc.2019.03.495
Henriksen PA (2018) Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 104(12):971–977. https://doi.org/10.1136/heartjnl-2017-312103
Barrios CHAA, Delaloge S, Montemurro F, Wuerstlein R, Robb S (2015) Safety of trastuzumab emtansine (T-DM1) in 373 patients 65 years or older with HER2-positive advanced breast cancer: a subgroup analysis of the Kamilla study. J Clin Oncol 33:603
Krop IE, Kim SB, Martin AG, LoRusso PM, Ferrero JM, Badovinac-Crnjevic T, Hoersch S, Smitt M, Wildiers H (2017) Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 18(6):743–754. https://doi.org/10.1016/S1470-2045(17)30313-3
Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, Martin M, Pienkowski T, Pivot X, Burris H 3rd, Petersen JA, Stanzel S, Strasak A, Patre M, Ellis P (2017) Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol 35(2):141–148. https://doi.org/10.1200/JCO.2016.67.4887
Funding
This research was supported through provision of data by Institute of Institute for Clinical Evaluative Sciences (ICES) and Cancer Care Ontario (CCO) and through funding support to ICES from an annual grant by the Ministry of Health and Long-Term Care (MOHLTC) and the Ontario Institute for Cancer Research (OICR). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES, CCO, OICR or the Government of Ontario is intended or should be inferred.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no actual or potential conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
As the data were de-identified in linked administrative databases accessed through ICES, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gong, I.Y., Yan, A.T., Earle, C.C. et al. Comparison of outcomes in a population-based cohort of metastatic breast cancer patients receiving anti-HER2 therapy with clinical trial outcomes. Breast Cancer Res Treat 181, 155–165 (2020). https://doi.org/10.1007/s10549-020-05614-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05614-5