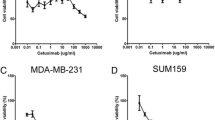
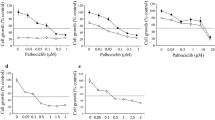
Abstract
Cancer stem cells (CSCs) are thought to be responsible for tumor progression, metastasis, and recurrence. HER2 overexpression is associated with increased CSCs, which may explain the aggressive phenotype and increased likelihood of recurrence for HER2+ breast cancers. Telomerase is reactivated in tumor cells, including CSCs, but has limited activity in normal tissues, providing potential for telomerase inhibition in anti-cancer therapy. The purpose of this study was to investigate the effects of a telomerase antagonistic oligonucleotide, imetelstat (GRN163L), on CSC and non-CSC populations of HER2+ breast cancer cell lines. The effects of imetelstat on CSC populations of HER2+ breast cancer cells were measured by ALDH activity and CD44/24 expression by flow cytometry as well as mammosphere assays for functionality. Combination studies in vitro and in vivo were utilized to test for synergism between imetelstat and trastuzumab. Imetelstat inhibited telomerase activity in both subpopulations. Moreover, imetelstat alone and in combination with trastuzumab reduced the CSC fraction and inhibited CSC functional ability, as shown by decreased mammosphere counts and invasive potential. Tumor growth rate was slower in combination-treated mice compared to either drug alone. Additionally, there was a trend toward decreased CSC marker expression in imetelstat-treated xenograft cells compared to vehicle control. Furthermore, the observed decrease in CSC marker expression occurred prior to and after telomere shortening, suggesting that imetelstat acts on the CSC subpopulation in telomere length-dependent and -independent mechanisms. Our study suggests addition of imetelstat to trastuzumab may enhance the effects of HER2 inhibition therapy, especially in the CSC population.






Similar content being viewed by others
References
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988. doi:10.1073/pnas.0530291100
Cho RW, Clarke MF (2008) Recent advances in cancer stem cells. Curr Opin Genet Dev 18(1):48–53. doi:10.1016/j.gde.2008.01.017
Wicha MS, Liu S, Dontu G (2006) Cancer stem cells: an old idea–a paradigm shift. Cancer Res 66 (4):1883–1890; discussion 1895–1886. doi:10.1158/0008-5472.can-05-3153
Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8(10):755–768. doi:10.1038/nrc2499
Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH, Cote RJ (2006) Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res 12(19):5615–5621. doi:10.1158/1078-0432.CCR-06-0169
Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG (2005) Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 65(13):5506–5511. doi:10.1158/0008-5472.can-05-0626
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100(9):672–679. doi:10.1093/jnci/djn123
Phillips TM, McBride WH, Pajonk F (2006) The response of CD24(−/low)/CD44 + breast cancer-initiating cells to radiation. J Natl Cancer Inst 98(24):1777–1785. doi:10.1093/jnci/djj495
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1(5):555–567. doi:10.1016/j.stem.2007.08.014
Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, Sozzi G, Fontanella E, Menard S, Tagliabue E (2009) Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res 15(6):2010–2021. doi:10.1158/1078-0432.ccr-08-1327
Korkaya H, Paulson A, Iovino F, Wicha MS (2008) HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 27(47):6120–6130. doi:10.1038/onc.2008.207
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23(16):3676–3685. doi:10.1200/JCO.2005.07.032
Korkaya H, Wicha MS (2013) HER2 and breast cancer stem cells: more than meets the eye. Cancer Res 73(12):3489–3493. doi:10.1158/0008-5472.can-13-0260
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW (1994) Specific association of human telomerase activity with immortal cells and cancer. Science (New York, NY) 266(5193):2011–2015
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science (New York, NY) 279(5349):349–352
Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14(4):501–513
Zhang X, Mar V, Zhou W, Harrington L, Robinson MO (1999) Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev 13(18):2388–2399
Shay JW, Wright WE (2006) Telomerase therapeutics for cancer: challenges and new directions. Nat Rev Drug Discov 5(7):577–584. doi:10.1038/nrd2081
Gryaznov SM, Jackson S, Dikmen G, Harley C, Herbert BS, Wright WE, Shay JW (2007) Oligonucleotide conjugate GRN163L targeting human telomerase as potential anticancer and antimetastatic agent. Nucleosides, Nucleotides Nucleic Acids 26(10–12):1577–1579. doi:10.1080/15257770701547271
Herbert BS, Pongracz K, Shay JW, Gryaznov SM (2002) Oligonucleotide N3′ → P5′ phosphoramidates as efficient telomerase inhibitors. Oncogene 21(4):638–642. doi:10.1038/sj.onc.1205064
Asai A, Oshima Y, Yamamoto Y, Uochi TA, Kusaka H, Akinaga S, Yamashita Y, Pongracz K, Pruzan R, Wunder E, Piatyszek M, Li S, Chin AC, Harley CB, Gryaznov S (2003) A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res 63(14):3931–3939
Herbert BS, Gellert GC, Hochreiter A, Pongracz K, Wright WE, Zielinska D, Chin AC, Harley CB, Shay JW, Gryaznov SM (2005) Lipid modification of GRN163, an N3′ → P5′ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene 24(33):5262–5268. doi:10.1038/sj.onc.1208760
Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, Dogan P, Wright WE, Shay JW (2005) In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res 65(17):7866–7873. doi:10.1158/0008-5472.can-05-1215
Dikmen ZG, Wright WE, Shay JW, Gryaznov SM (2008) Telomerase targeted oligonucleotide thio-phosphoramidates in T24-luc bladder cancer cells. J Cell Biochem 104(2):444–452. doi:10.1002/jcb.21635
Djojosubroto MW, Chin AC, Go N, Schaetzlein S, Manns MP, Gryaznov S, Harley CB, Rudolph KL (2005) Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatoma. Hepatology (Baltimore, Md) 42 (5):1127–1136. doi:10.1002/hep.20822
Goldblatt EM, Erickson PA, Gentry ER, Gryaznov SM, Herbert BS (2009) Lipid-conjugated telomerase template antagonists sensitize resistant HER2-positive breast cancer cells to trastuzumab. Breast Cancer Res Treat 118(1):21–32. doi:10.1007/s10549-008-0201-4
Goldblatt EM, Gentry ER, Fox MJ, Gryaznov SM, Shen C, Herbert BS (2009) The telomerase template antagonist GRN163L alters MDA-MB-231 breast cancer cell morphology, inhibits growth, and augments the effects of paclitaxel. Mol Cancer Ther 8(7):2027–2035. doi:10.1158/1535-7163.mct-08-1188
Gomez-Millan J, Goldblatt EM, Gryaznov SM, Mendonca MS, Herbert BS (2007) Specific telomere dysfunction induced by GRN163L increases radiation sensitivity in breast cancer cells. Int J Radiat Oncol Biol Phys 67(3):897–905. doi:10.1016/j.ijrobp.2006.09.038
Hochreiter AE, Xiao H, Goldblatt EM, Gryaznov SM, Miller KD, Badve S, Sledge GW, Herbert BS (2006) Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clin Cancer Res 12(10):3184–3192. doi:10.1158/1078-0432.ccr-05-2760
Shammas MA, Koley H, Bertheau RC, Neri P, Fulciniti M, Tassone P, Blotta S, Protopopov A, Mitsiades C, Batchu RB, Anderson KC, Chin A, Gryaznov S, Munshi NC (2008) Telomerase inhibitor GRN163L inhibits myeloma cell growth in vitro and in vivo. Leukemia 22(7):1410–1418. doi:10.1038/leu.2008.81
Burchett KM, Yan Y, Ouellette MM (2014) Telomerase inhibitor Imetelstat (GRN163L) limits the lifespan of human pancreatic cancer cells. PLoS ONE 9(1):e85155. doi:10.1371/journal.pone.0085155
Ju Z, Rudolph KL (2006) Telomeres and telomerase in cancer stem cells. Eur J Cancer (Oxford, England : 1990) 42 (9):1197–1203. doi:10.1016/j.ejca.2006.01.040
Castelo-Branco P, Zhang C, Lipman T, Fujitani M, Hansford L, Clarke I, Harley CB, Tressler R, Malkin D, Walker E, Kaplan DR, Dirks P, Tabori U (2011) Neural tumor-initiating cells have distinct telomere maintenance and can be safely targeted for telomerase inhibition. Clin Cancer Res 17(1):111–121. doi:10.1158/1078-0432.CCR-10-2075
Joseph I, Tressler R, Bassett E, Harley C, Buseman CM, Pattamatta P, Wright WE, Shay JW, Go NF (2010) The telomerase inhibitor imetelstat depletes cancer stem cells in breast and pancreatic cancer cell lines. Cancer Res 70(22):9494–9504. doi:10.1158/0008-5472.can-10-0233
Marian CO, Wright WE, Shay JW (2010) The effects of telomerase inhibition on prostate tumor-initiating cells. Int J Cancer 127(2):321–331. doi:10.1002/ijc.25043
Marian CO, Cho SK, McEllin BM, Maher EA, Hatanpaa KJ, Madden CJ, Mickey BE, Wright WE, Shay JW, Bachoo RM (2010) The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth. Clin Cancer Res 16(1):154–163. doi:10.1158/1078-0432.ccr-09-2850
Ruden M, Puri N (2013) Novel anticancer therapeutics targeting telomerase. Cancer Treat Rev 39(5):444–456. doi:10.1016/j.ctrv.2012.06.007
Nahta R, Esteva FJ (2004) In vitro effects of trastuzumab and vinorelbine in trastuzumab-resistant breast cancer cells. Cancer Chemother Pharmacol 53(2):186–190. doi:10.1007/s00280-003-0728-3
Helbig G, Christopherson KW 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H (2003) NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 278(24):21631–21638. doi:10.1074/jbc.M300609200
Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS (2009) Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 69(4):1302–1313. doi:10.1158/0008-5472.can-08-2741
Fillmore CM, Kuperwasser C (2008) Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 10(2):R25. doi:10.1186/bcr1982
O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, Duffy MJ, Crown J, O’Donovan N, Slamon DJ (2010) Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther 9(6):1489–1502. doi:10.1158/1535-7163.mct-09-1171
Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R Jr, Badve S, Nakshatri H (2006) CD44 +/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 8(5):R59. doi:10.1186/bcr1610
Chen D, Bhat-Nakshatri P, Goswami C, Badve S, Nakshatri H (2013) ANTXR1, a stem cell-enriched functional biomarker, connects collagen signaling to cancer stem-like cells and metastasis in breast cancer. Cancer Res 73(18):5821–5833. doi:10.1158/0008-5472.CAN-13-1080
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715. doi:10.1016/j.cell.2008.03.027
Brennan SK, Wang Q, Tressler R, Harley C, Go N, Bassett E, Huff CA, Jones RJ, Matsui W (2010) Telomerase inhibition targets clonogenic multiple myeloma cells through telomere length-dependent and independent mechanisms. PLoS ONE. doi:10.1371/journal.pone.0012487
Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES (2009) Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138(4):645–659. doi:10.1016/j.cell.2009.06.034
Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 3(8):e2888. doi:10.1371/journal.pone.0002888
Tsai JH, Yang J (2013) Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 27(20):2192–2206. doi:10.1101/gad.225334.113
Mender I, Senturk S, Ozgunes N, Akcali KC, Kletsas D, Gryaznov S, Can A, Shay JW, Dikmen ZG (2013) Imetelstat (a telomerase antagonist) exerts offtarget effects on the cytoskeleton. Int J Oncol 42(5):1709–1715. doi:10.3892/ijo.2013.1865
Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, Hu GF (2008) Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res 68(24):10377–10386. doi:10.1158/0008-5472.can-08-1444
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD, Hong S, Adams A, D’Angelo R, Ginestier C, Charafe-Jauffret E, Clouthier SG, Birnbaum D, Wong ST, Zhan M, Chang JC, Wicha MS (2014) Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem cell reports 2(1):78–91. doi:10.1016/j.stemcr.2013.11.009
Biddle A, Liang X, Gammon L, Fazil B, Harper LJ, Emich H, Costea DE, Mackenzie IC (2011) Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res 71(15):5317–5326. doi:10.1158/0008-5472.CAN-11-1059
Acknowledgments
We would like to thank the members of the Herbert Lab for helpful discussion preparing this manuscript. We thank Geron Corporation for generously providing the imetelstat and sense oligonucleotides, Dr. Francisco Esteva for kindly sharing the SKBR3 and SKBR3-R cell lines, Dr. Harikrishna Nakshatri for kindly sharing the TMD-231 cell line, Dr. Harlan Shannon for help with the combination studies, Dr. George Sandusky for help with histology, Dr. Hiromi Tanaka for help with TeloTAGGG analysis, the Indiana University Simon Cancer Center (IUSCC) flow cytometry core facility for their services and expertise, and the IUSCC infusion pharmacy for generously providing the trastuzumab. This investigator was supported, in part, by the National Institutes of Health, National Research Service Award Number T32 HL007910- Basic Science Studies on Gene Therapy of Blood Diseases. This work was also supported in part by a Grant from Susan G. Komen for the Cure®, an IUSCC Cancer Biology Training Program Predoctoral Fellowship, a grant from the Mary Kay Ash Charitable Foundation, and the Indiana Genomics Initiative (INGEN; supported in part by the Lilly Endowment, Inc). We are also grateful for the philanthropic support made to the Herbert laboratory through IUSCC in memory of Carol Herbert.
Conflict of interest
Imetelstat and sense oligonucleotides were generously provided by the Geron Corporation. The authors declare they have no other conflicts of interest.
Ethical standards
The authors declare that the experiments performed comply with the current laws.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koziel, J.E., Herbert, BS. The telomerase inhibitor imetelstat alone, and in combination with trastuzumab, decreases the cancer stem cell population and self-renewal of HER2+ breast cancer cells. Breast Cancer Res Treat 149, 607–618 (2015). https://doi.org/10.1007/s10549-015-3270-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3270-1