Abstract
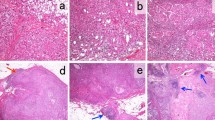
The anti-tumor immune response was recently reported to play a critical role in the chemotherapeutic sensitivity of breast cancer. Therefore, we investigated the correlation between CD8+ and FOXP3+ tumor-infiltrating lymphocytes and the pathological complete response (pCR) following neoadjuvant chemotherapy (NAC) in triple-negative breast cancer (TNBC), in conjunction with neoangiogenesis, basal and proliferation markers. CD8+ and FOXP3+ lymphocytes were assessed in biopsy specimens by double-staining immunohistochemistry, in combination with immunostaining of vasohibin-1, CD31, EGFR, CK5/6, and Ki-67. Earlier age, pre-menopausal status, smaller tumor size, and high Ki-67 were significantly associated with pCR, as in high CD8+, high CD8+/FOXP3+ ratio, and low vasohibin-1 positive ratio. Multivariate analysis did reveal that a high CD8+/FOXP3+ ratio was a strong predictor of pCR with an odds ratio of 5.32 (P = 0.005). High Ki-67 was also significantly associated with pCR (P = 0.002). TNBCs with a high CD8+/FOXP3+ ratio and high Ki-67 had the highest pCR rate (70 %) following NAC. However, the pCR rate of the patients with low CD8+/FOXP3+ ratio and low Ki-67 was only 5 %. The pCR rates of a high CD8+/FOXP3+ ratio and low Ki-67 patients and those with a low CD8+/FOXP3+ ratio and high Ki-67 were 24 and 21 %, respectively. TNBCs with a high CD8+/FOXP3+ ratio were more sensitive to anthracycline and taxane-based chemotherapeutic regimens, and the CD8+/FOXP3+ ratio in conjunction with Ki-67 could predict pCR following NAC in TNBC. This predictor may represent a new surrogate for testing the efficacy of investigational agents in the neoadjuvant setting.





Similar content being viewed by others
References
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Sorlie T, Tibshirani R, Parker J et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
Liedtke C, Mazouni C, Hess KR et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281
Darb-Esfahani S, Loibl S, Muller BM et al (2009) Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res 11:R69
Denkert C, Loibl S, Muller BM et al (2013) Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol 24:2786–2793
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig 121:2750–2767
Masuda H, Baggerly KA, Wang Y et al (2013) Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 19:5533–5540
Apetoh L, Ghiringhelli F, Tesniere A et al (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059
Andre F, Dieci MV, Dubsky P et al (2013) Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res 19:28–33
Liu F, Lang R, Zhao J et al (2011) CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat 130:645–655
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO (2012) CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res 14:R48
Ali HR, Provenzano E, Dawson SJ et al (2014) Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann Oncol 25:1536–1543
Liyanage UK, Moore TT, Joo HG et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761
Viguier M, Lemaitre F, Verola O et al (2004) Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 173:1444–1453
Mahmoud SM, Paish EC, Powe DG et al (2011) An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat 127:99–108
Oda N, Shimazu K, Naoi Y et al (2012) Intratumoral regulatory T cells as an independent predictive factor for pathological complete response to neoadjuvant paclitaxel followed by 5-FU/epirubicin/cyclophosphamide in breast cancer patients. Breast Cancer Res Treat 136:107–116
West NR, Kost SE, Martin SD et al (2013) Tumour-infiltrating FOXP3+ lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer 108:155–162
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Weidner N, Folkman J, Pozza F et al (1992) Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 84:1875–1887
Bevilacqua P, Barbareschi M, Verderio P et al (1995) Prognostic value of intratumoral microvessel density, a measure of tumor angiogenesis, in node-negative breast carcinoma—results of a multiparametric study. Breast Cancer Res Treat 36:205–217
Chan MS, Wang L, Chanplakorn N et al (2012) Effects of estrogen depletion on angiogenesis in estrogen-receptor-positive breast carcinoma—an immunohistochemical study of vasohibin-1 and CD31 with correlation to pathobiological response of the patients in neoadjuvant aromatase inhibitor therapy. Expert Opin Ther Targets 16(Suppl 1):S69–S78
Fu J, Xu D, Liu Z et al (2007) Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132:2328–2339
Mahmoud SM, Paish EC, Powe DG et al (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29:1949–1955
Tamaki K, Moriya T, Sato Y et al (2009) Vasohibin-1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci 100:88–94
Tamaki K, Sasano H, Maruo Y et al (2010) Vasohibin-1 as a potential predictor of aggressive behavior of ductal carcinoma in situ of the breast. Cancer Sci 101:1051–1058
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Urruticoechea A, Smith IE, Dowsett M (2005) Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23:7212–7220
de Azambuja E, Cardoso F, de Castro Jr G et al (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96:1504–1513
Lerma E, Peiro G, Ramon T et al (2007) Immunohistochemical heterogeneity of breast carcinomas negative for estrogen receptors, progesterone receptors and Her2/neu (basal-like breast carcinomas). Mod Pathol 20:1200–1207
Rakha EA, Reis-Filho JS, Ellis IO (2008) Basal-like breast cancer: a critical review. J Clin Oncol 26:2568–2581
Bertucci F, Finetti P, Rougemont J et al (2005) Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res 65:2170–2178
Bertucci F, Finetti P, Cervera N et al (2006) Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res 66:4636–4644
Ladoire S, Arnould L, Apetoh L et al (2008) Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res 14:2413–2420
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalized the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223
Doedens AL, Stockmann C, Rubinstein MP et al (2010) Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 70:7465–7475
Tartour E, Pere H, Maillere B et al (2011) Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev 30:83–95
Acknowledgments
We wish to thank Yayoi Takahashi, MT, for her excellent technical assistance. This work was partly supported by a Grant-in-Aid for researches on the construction of treatment algorithm in triple-negative breast cancer (No. 26830094) from the Japan Society for the Promotion of Science.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Figure Pathological complete response (pCR) rates of TNBC tumors by the clinicopathological factors, including basal-like status, Ki-67 labeling index (LI), Vasohibin-1, CD31 and vasohibin-1 positive ratio (VPR). HG: histological grade.
Rights and permissions
About this article
Cite this article
Miyashita, M., Sasano, H., Tamaki, K. et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat 148, 525–534 (2014). https://doi.org/10.1007/s10549-014-3197-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3197-y