Abstract
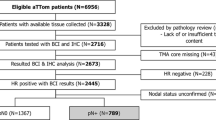
BAG1 is a multifunctional anti-apoptotic protein located on chromosome 9q12, which binds to Bcl-2. BAG1 is present as a separate module in the GHI-RS 21-gene panel. It may provide additional prognostic information as an immunohistochemical marker when added to IHC4. Analysis of BAG1 was performed on archival tumour blocks from patients from the anastrozole and tamoxifen arms of the ATAC trial of 5 years endocrine therapy in postmenopausal women with oestrogen receptor (ER)-positive primary breast cancer. Staining was scored separately as nuclear or cytoplasmic. Statistical analyses were performed on data from median 10-year follow-up with distant recurrence as primary endpoint. Data on both nuclear and cytoplasmic BAG1 as well as the IHC4 markers (ER, PgR, HER2 and Ki67) were available on 963 ER-positive cases of which 860 were HER2-negative. Cytoplasmic and nuclear BAG1 were highly correlated (Spearman r = 0.79, p < 00001). Women with higher BAG1 expression developed 30 % fewer distant recurrences compared to those with low expression. Nuclear BAG1 contributed significantly to the clinical and IHC4 models with added information being greater in node-positive cases. Similar results were seen if all recurrences were the endpoints. BAG1 expression provides significant prognostic information when added to the classical clinicopathological parameters and IHC4, particularly in node-positive patients.



Similar content being viewed by others
References
Dowsett M, Goldhirsch A, Hayes DF et al (2007) International Web-based consultation on priorities for translational breast cancer research. Breast Cancer Res 9(6):R81
Cuzick J, Sestak I, Baum M et al (2010) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 11(12):1135–1141
Dowsett M, Cuzick J, Wale C et al (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28(11):1829–1834
Tang SC, Beck J, Murphy S et al (2004) BAG-1 expression correlates with Bcl-2, p53, differentiation, estrogen and progesterone receptors in invasive breast carcinoma. Breast Cancer Res Treat 84(3):203–213
Dowsett M, Allred C, Knox J et al (2008) Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the arimidex, tamoxifen, alone or in combination trial. J Clin Oncol 26(7):1059–1065
Cuzick J, Dowsett M, Pineda S et al (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the genomic health recurrence score in early breast cancer. J Clin Oncol 29(32):4273–4278
Christiansen J, Bartlett JMS, Gustavson M et al. (2012) Validation of IHC4 algorithms for prediction of risk of recurrence in early breast cancer using both conventional and quantitative IHC approaches. J Clin Oncol 30 (suppl: abstract 517):2977–2298
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
Townsend PA, Stephanou A, Packham G et al (2005) BAG-1: a multi-functional pro-survival molecule. Int J Biochem Cell Biol 37(2):251–259
Townsend PA, Dublin E, Hart IR et al (2002) BAG-I expression in human breast cancer: interrelationship between BAG-1 RNA, protein, HSC70 expression and clinico-pathological data. J Pathol 197(1):51–59
Nadler Y, Camp RL, Giltnane JM et al (2008) Expression patterns and prognostic value of Bag-1 and Bcl-2 in breast cancer. Breast Cancer Res 10(2):R35
Tang SC (2002) BAG-1, an anti-apoptotic tumour marker. IUBMB Life 53(2):99–105
Millar EK, Anderson LR, McNeil CM et al (2009) BAG-1 predicts patient outcome and tamoxifen responsiveness in ER-positive invasive ductal carcinoma of the breast. Br J Cancer 100(1):123–133
Gee JM, Aleskandarany MA, Finlay P et al. (2010) Immunohistochemical markers progesterone, HER2, Ki67 and Bcl-2-associated anthogene 1 and prediction of adjuvant tamoxifen treatment outcome in ER+ early breast cancer In: San Antonio breast cancer symposium, San Antonio
Barton S, Zabaglo L, A’Hern R et al (2012) Assessment of the contribution of the IHC4+C score to decision making in clinical practice in early breast cancer. Br J Cancer 106(11):1760–1765
Harper-Wynne CL, Sacks NP, Shenton K et al (2002) Comparison of the systemic and intratumoral effects of tamoxifen and the aromatase inhibitor vorozole in postmenopausal patients with primary breast cancer. J Clin Oncol 20(4):1026–1035
Petry IB, Fieber E, Schmidt M et al (2010) ERBB2 induces an antiapoptotic expression pattern of Bcl-2 family members in node-negative breast cancer. Clin Cancer Res 16(2):451–460
Cutress RI, Townsend PA, Sharp A et al (2003) The nuclear BAG-1 isoform, BAG-1L, enhances oestrogen-dependent transcription. Oncogene 22(32):4973–4982
Acknowledgments
This study is supported by the Royal Marsden National Institute for Health Research Biomedical Research Centre, Cancer Research UK Programme Grant No: C569-10404, and Grants from Breakthrough Breast Cancer and AstraZeneca. The authors are grateful to Professor Michael Baum for his guidance and advice throughout the ATAC trial.
Disclosures
JC receives trial support and sits on an advisory board for AstraZeneca. MD has received lecture fees and research grants from AstraZeneca in the last 2 years. All remaining authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2013_2628_MOESM1_ESM.xls
Supplementary Table 1: Univariate and multivariate analysis of cytoplasmic and nuclear BAG1 (considered as positive or negative). (XLS 26 kb)
10549_2013_2628_MOESM2_ESM.xlsx
Supplementary Table 2: Univariate and multivariate analysis of cytoplasmic and nuclear BAG1 (considered as positive or negative). (XLSX 41 kb)
Rights and permissions
About this article
Cite this article
Afentakis, M., Dowsett, M., Sestak, I. et al. Immunohistochemical BAG1 expression improves the estimation of residual risk by IHC4 in postmenopausal patients treated with anastrazole or tamoxifen: a TransATAC study. Breast Cancer Res Treat 140, 253–262 (2013). https://doi.org/10.1007/s10549-013-2628-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2628-5